Amidoxime Group on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 Gentler oxidants give mono-nitro compounds.
In the
Gentler oxidants give mono-nitro compounds.
In the 
 Oximes are commonly used as ligands and sequestering agents for metal ions.
Oximes are commonly used as ligands and sequestering agents for metal ions.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, an oxime is an organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
belonging to the imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s, with the general formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
, where R is an organic side-chain and R' may be hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, forming an aldoxime, or another organic group
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s () with general structure .
Oximes are usually generated by the reaction of hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Prof ...
with aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s () or ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''.
Structure and properties
If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometricstereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
ic forms according to the ''E''/''Z'' configuration. An older terminology of ''syn'' and ''anti'' was used to identify especially aldoximes according to whether the R group was closer or further from the hydroxyl. Both forms are often stable enough to be separated from each other by standard techniques.
Oximes have three characteristic bands in the infrared spectrum
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those of ...
, whose wavelengths corresponding to the stretching vibrations of its three types of bonds: 3600 cm−1 (O−H), 1665 cm−1 (C=N) and 945 cm−1 (N−O).
In aqueous solution, aliphatic oximes are 102- to 103-fold more resistant to hydrolysis than analogous hydrazones.
Preparation
Oximes can be synthesized bycondensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of an aldehyde or a ketone with hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Prof ...
. The condensation of aldehydes with hydroxylamine gives aldoximes, and ketoximes are produced from ketones and hydroxylamine. In general, oximes exist as colorless crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s or as thick liquids and are poorly soluble in water. Therefore, oxime formation can be used for the identification of ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
or aldehyde functional groups.
Oximes can also be obtained from reaction of nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name ...
s such as isoamyl nitrite
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group (the amyl in this case) is unreactive and the chemical ...
with compounds containing an acidic hydrogen atom. Examples are the reaction of ethyl acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds.
Preparation
At large scale, ethyl ac ...
and sodium nitrite
Sodium nitrite is an inorganic compound with the chemical formula . It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite sa ...
in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, the reaction of methyl ethyl ketone
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large s ...
with ethyl nitrite
The chemical compound ethyl nitrite is an alkyl nitrite with a chemical formula C2H5NO2. It may be prepared from ethanol.
:
Uses
It is used as a reagent with butanone to yield the dimethylglyoxime end product.
:
Ethyl nitrite is the main in ...
in hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
. and a similar reaction with propiophenone
Propiophenone (shorthand: benzoylethane or BzEt) is an aryl ketone. It is a colorless, sweet-smelling liquid that is insoluble in water, but miscible with organic solvents. It is used in the preparation of other compounds.
Production
Propiophenon ...
, the reaction of phenacyl chloride
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It ...
, the reaction of malononitrile
Malononitrile is an organic compound nitrile with the formula . It is a colorless or white solid, although aged samples appear yellow or even brown. It is a widely used building block in organic synthesis.
Preparation and reactions
It can be pre ...
with sodium nitrite in acetic acid
A conceptually related reaction is the Japp–Klingemann reaction
The Japp–Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids (or β-keto-esters) and aryl diazonium salts. The reaction is named after the chemists Francis Robert Japp and Felix Klingemann.
:
The hydrazon ...
.
Reactions
Thehydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of oximes proceeds easily by heating in the presence of various inorganic acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water.
Ch ...
s, and the oximes decompose into the corresponding ketones or aldehydes, and hydroxylamines. The reduction of oximes by sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
metal, sodium amalgam
Sodium amalgam, with the common formula Na(Hg), is an alloy of mercury and sodium. The term amalgam is used for alloys, intermetallic compounds, and solutions (both solid solutions and liquid solutions) involving mercury as a major component. ...
, hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated ...
, or reaction with hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
reagents produces amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s. Typically the reduction of aldoximes gives both primary amines and secondary amines; however, reaction conditions can be altered (such as the addition of potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which utili ...
in a 1/30 molar ratio) to yield solely primary amines.
In general, oximes can be changed to the corresponding amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
derivatives by treatment with various acids. This reaction is called Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
. In this reaction, a hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
is exchanged with the group that is in the anti position of the hydroxyl group. The amide derivatives that are obtained by Beckmann rearrangement can be transformed into a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
by means of hydrolysis (base or acid catalyzed). Beckmann rearrangement is used for the industrial synthesis of caprolactam
Caprolactam (CPL) is an organic compound with the chemical formula, formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast ...
(see applications below).
The Ponzio reaction (1906) concerning the conversion of ''m''-nitrobenzaldoxime to ''m''-nitrophenyldinitromethane using dinitrogen tetroxide
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russian rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium ...
was the result of research into TNT
Troponin T (shortened TnT or TropT) is a part of the troponin complex, which are proteins integral to the contraction of skeletal and heart muscles. They are expressed in skeletal and cardiac myocytes. Troponin T binds to tropomyosin and helps ...
analogues:
Neber rearrangement The Neber rearrangement is an organic reaction in which a ketoxime is converted into an alpha- aminoketone via a rearrangement reaction.
:
The oxime is first converted to an O-sulfonate, for example a tosylate by reaction with tosyl chloride. Adde ...
certain oximes are converted to the corresponding alpha-amino ketones.
Oximes can be dehydrated
In physiology, dehydration is a lack of total body water that disrupts Metabolism, metabolic processes. It occurs when free water loss exceeds intake, often resulting from excessive sweating, health conditions, or inadequate consumption of wate ...
using acid anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one equivalent of wa ...
s to yield corresponding nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The name of the compound is composed of a base, which includes the carbon of the , suffixed with "nitrile", so for example is called " propionitrile" (or pr ...
s.
Certain amidoximes react with benzenesulfonyl chloride
Benzenesulfonyl chloride is an organosulfur compound with the formula C6H5SO2Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfo ...
to make substituted urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
s in the Tiemann rearrangement:
:
Uses
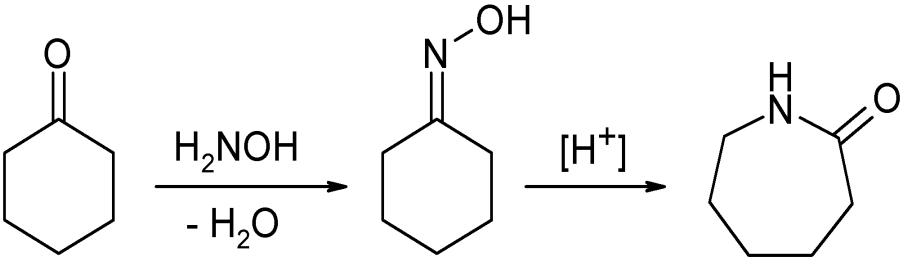
In their largest application, an oxime is an intermediate in the industrial production ofcaprolactam
Caprolactam (CPL) is an organic compound with the chemical formula, formula (CH2)5C(O)NH. This colourless solid is a lactam (a cyclic amide) of caproic acid. Global demand for this compound is approximately five million tons per year, and the vast ...
, a precursor to Nylon 6
Nylon 6 or polycaprolactam is a polymer, in particular semicrystalline polyamide. Unlike most other nylons, nylon 6 is not a condensation polymer, but instead is formed by ring-opening polymerization; this makes it a special case in the compari ...
. About half of the world's supply of cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
, more than a million tonnes annually, is converted to the oxime. In the presence of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
, the oxime undergoes the Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
to give the cyclic amide caprolactam:
::
Metal extractant
 Oximes are commonly used as ligands and sequestering agents for metal ions.
Oximes are commonly used as ligands and sequestering agents for metal ions. Dimethylglyoxime
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH− for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the dike ...
(dmgH2) is a reagent for the analysis of nickel and a popular ligand in its own right. In the typical reaction, a metal reacts with two equivalents of dmgH2 concomitant with ionization of one proton. Salicylaldoxime
Salicylaldoxime is an organic compound described by the formula C6H4CH=NOH-2-OH. It is the oxime of salicylaldehyde. This crystalline, colorless solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, ...
is a chelator
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
in hydrometallurgy
Hydrometallurgy is a technique within the field of extractive metallurgy, the obtaining of metals from their ores. Hydrometallurgy uses solutions to recover metals from ores, concentrates, and recycled or residual materials. Usually the extracti ...
.
Amidoximes such as polyacrylamidoxime can be used to capture trace amounts of uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
from sea water. In 2017 researchers announced a configuration that absorbed up to nine times as much uranyl
The uranyl ion with the chemical formula has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen, with uranium in the oxidation state +6. Four or more ligands may be bound to the u ...
as previous fibers without saturating.
Other applications
* Oxime compounds are used as antidotes fornerve agent
Nerve agents, sometimes also called nerve gases, are a class of organic chemistry, organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (ACh ...
s. A nerve agent inactivates acetylcholinesterase
Acetylcholinesterase (HUGO Gene Nomenclature Committee, HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme th ...
by phosphorylation. Oxime compounds can reactivate acetylcholinesterase by attaching to phosphorus, forming an oxime-phosphonate, which then splits away from the acetylcholinesterase molecule. Oxime nerve-agent antidotes are pralidoxime
Pralidoxime (2-pyridine aldoxime methyl chloride) or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to treat organophosph ...
(also known as 2-PAM
Pralidoxime (2-pyridine aldoxime methyl chloride) or 2-PAM, usually as the chloride or iodide salts, belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to treat organophospha ...
), obidoxime
Obidoxime is a member of the oxime family used to treat organophosphate poisoning. Oximes are drugs known for their ability to reverse the binding of organophosphorus compounds to the enzyme acetylcholinesterase (AChE).
AChE is an enzyme that ...
, methoxime, HI-6, Hlo-7, and TMB-4. The effectiveness of the oxime treatment depends on the particular nerve agent used.
* Perillartine
Perillartine, also known as perillartin and perilla sugar, is a semisynthetic sweetener that is about 2000 times as sweet as sucrose. It is mainly used in Japan.perillaldehyde
Perillaldehyde, perillic aldehyde or perilla aldehyde, is a natural organic compound found most abundantly in the annual herb perilla, but also in a wide variety of other plants and essential oils. It is a monoterpenoid containing an aldehyde f ...
, is used as an artificial sweetener in Japan. It is 2000 times sweeter than sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
.
* Diaminoglyoxime is a key precursor to various compounds containing the highly reactive furazan
Furazan, or 1,2,5-oxadiazole, is a heterocyclic aromatic organic compound consisting of a five-atom ring containing 1 oxygen and 2 nitrogen atoms. The furazan ring system is also found in the steroid furazabol. Furazan and its derivatives are ob ...
ring.
* Methyl ethyl ketoxime is a skin-preventing additive in many oil-based paints.
* Buccoxime and 5-methyl-3-heptanone oxime ("Stemone") are perfume ingredients.Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim.
*Fluvoxamine
Fluvoxamine, sold under the brand name Luvox among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is primarily used to treat major depressive disorder and, perhaps more-especially, obsessive–compu ...
is used as an antidepressant.
See also
* :Oximes – specific chemicals containing this functional group *Nitrone
In organic chemistry, a nitrone is a functional group consisting of an Amine oxide, ''N''-oxide of an imine. The general structure is , where R3 is not a hydrogen. Their primary application is Chemical Intermediate, intermediates in chemical syn ...
– the ''N''-oxide of an imine
References
{{Authority control Functional groups Organic compounds Chelating agents