Alpha Elements on:
[Wikipedia]
[Google]
[Amazon]
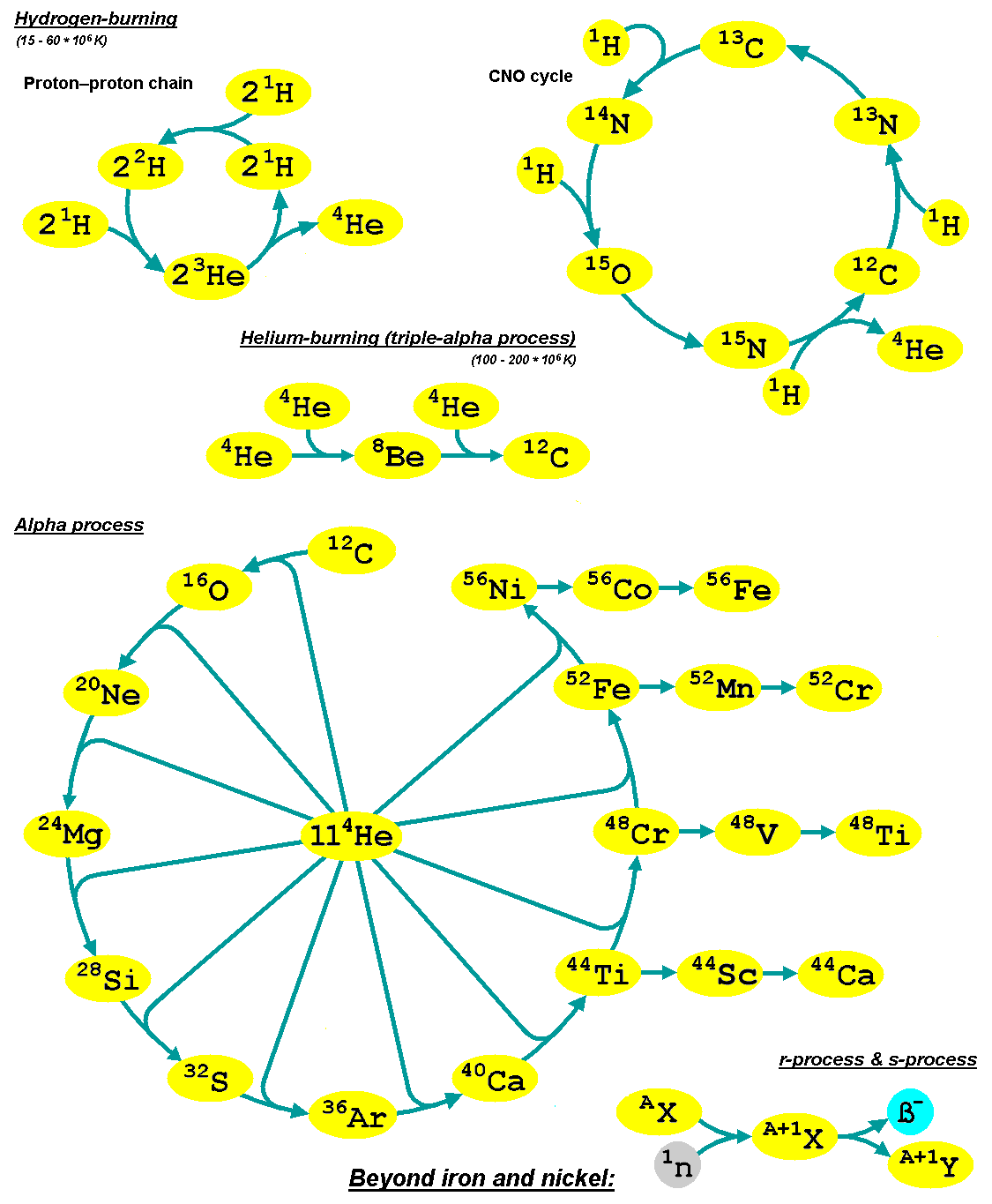 The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of  It is a common misconception that the above sequence ends at (or , which is a decay product of ) because it is the most tightly bound
It is a common misconception that the above sequence ends at (or , which is a decay product of ) because it is the most tightly bound
 * The stable alpha elements are: C, O, Ne, Mg, Si, and S.
* The elements Ar and Ca are ''"
* The stable alpha elements are: C, O, Ne, Mg, Si, and S.
* The elements Ar and Ca are ''"
 The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of
The alpha process, also known as alpha capture or the alpha ladder, is one of two classes of nuclear fusion
Nuclear fusion is a nuclear reaction, reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutrons, neutron by-products. The difference in mass between the reactants and products is manifested as either the rele ...
reactions by which stars convert helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
into heavier elements. The other class is a cycle of reactions called the triple-alpha process
The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.
In stars
Helium accumulates in the cores of stars as a result of the proton–proton chain reaction a ...
, which consumes only helium, and produces carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
. The alpha process most commonly occurs in massive stars and during supernovae
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion. The original ob ...
.
Both processes are preceded by hydrogen fusion
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
, which produces the helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
that fuels both the triple-alpha process and the alpha ladder processes. After the triple-alpha process
The triple-alpha process is a set of nuclear fusion reactions by which three helium-4 nuclei (alpha particles) are transformed into carbon.
In stars
Helium accumulates in the cores of stars as a result of the proton–proton chain reaction a ...
has produced enough carbon, the alpha-ladder begins and fusion reactions of increasingly heavy elements take place, in the order listed below. Each step only consumes the product of the previous reaction and helium. The later-stage reactions which are able to begin in any particular star, do so while the prior stage reactions are still under way in outer layers of the star.
:
The energy produced by each reaction, , is mainly in the form of gamma ray
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
s (), with a small amount taken by the byproduct
A by-product or byproduct is a secondary product derived from a production process, manufacturing process or chemical reaction; it is not the primary product or service being produced.
A by-product can be useful and marketable or it can be cons ...
element, as added momentum
In Newtonian mechanics, momentum (: momenta or momentums; more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. ...
.
nuclide
Nuclides (or nucleides, from nucleus, also known as nuclear species) are a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by the A ...
– i.e., the nuclide with the highest nuclear binding energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is alwa ...
per nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number.
Until the 1960s, nucleons were thought to be ele ...
– and production of heavier nuclei would consume energy (be endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
) instead of release it (exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
). (Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons.
It is a stable isotope, with the highest binding energy per nucleon of any known nuclide (8.7945 MeV). It is often stated that 56Fe is the "most stable nucleus", but only beca ...
) is actually the most tightly bound nuclide in terms of binding energy (though has a lower energy or mass per nucleon). The reaction is actually exothermic, and indeed adding alphas continues to be exothermic all the way to , but nonetheless the sequence does effectively end at iron. The sequence stops before producing elements heavier than nickel because conditions in stellar interiors cause the competition between photodisintegration
Photodisintegration (also called phototransmutation, or a photonuclear reaction) is a nuclear process in which an atomic nucleus absorbs a high-energy gamma ray, enters an excited state, and immediately decays by emitting a subatomic particle. The ...
and the alpha process to favor photodisintegration around iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
. This leads to more being produced than
All these reactions have a very low rate at the temperatures and densities in stars and therefore do not contribute significant energy to a star's total output. They occur even less easily with elements heavier than neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
() due to the increasing Coulomb barrier
The Coulomb barrier, named after Coulomb's law, which is in turn named after physicist Charles-Augustin de Coulomb, is the energy barrier due to electrostatic interaction that two nuclei need to overcome so they can get close enough to undergo a ...
.
Alpha process elements
Alpha process elements (or alpha elements) are so-called since their most abundant isotopes are integer multiples of four – the mass of the helium nucleus (thealpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
). These isotopes are called ''alpha nuclide
An alpha nuclide is a nuclide that consists of an integer number of alpha particles. Alpha nuclides have equal, even numbers of protons and neutrons; they are important in stellar nucleosynthesis since the energetic environment within stars is ame ...
s''.
observationally stable
Stable nuclides are isotopes of a chemical element whose nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The nuclei of such isotopes are not radioactive and unlike radionuc ...
"''. They are synthesized by alpha capture prior to the silicon fusing stage, that leads to
* Si and Ca are purely alpha process elements.
* Mg can be separately consumed by proton capture
Proton capture is a nuclear reaction in which an atomic nucleus and one or more protons collide and merge to form a heavier nucleus.
Since protons have positive electric charge, they are repelled electrostatically by the positively charged nucleu ...
reactions.
The status of oxygen ( O) is contested – some authors consider it an alpha element, while others do not. O is surely an alpha element in low-metallicity
In astronomy, metallicity is the Abundance of the chemical elements, abundance of Chemical element, elements present in an object that are heavier than hydrogen and helium. Most of the normal currently detectable (i.e. non-Dark matter, dark) matt ...
Population II star
In 1944, Walter Baade categorized groups of stars within the Milky Way into stellar populations.
In the abstract of the article by Baade, he recognizes that Jan Oort originally conceived this type of classification in 1926.
Baade observed that ...
s: It is produced in Type II supernovae, and its enhancement is well correlated with an enhancement of other alpha process elements.
Sometimes C and N are considered alpha process elements since, like O, they are synthesized in nuclear alpha-capture reactions, but their status is ambiguous: Each of the three elements is produced (and consumed) by the CNO cycle
In astrophysics, the carbon–nitrogen–oxygen (CNO) cycle, sometimes called Bethe–Weizsäcker cycle, after Hans Albrecht Bethe and Carl Friedrich von Weizsäcker, is one of the two known sets of fusion reactions by which stars convert h ...
, which can proceed at temperatures far lower than those where the alpha-ladder processes start producing significant amounts of alpha elements (including C, N, & O). So just the presence of C, N, or O in a star does not a clearly indicate that the alpha process is actually underway – hence reluctance of some astronomers to (unconditionally) call these three "alpha elements".
Production in stars
The alpha process generally occurs in large quantities only if the star is sufficiently massive – more massive than about 10solar mass
The solar mass () is a frequently used unit of mass in astronomy, equal to approximately . It is approximately equal to the mass of the Sun. It is often used to indicate the masses of other stars, as well as stellar clusters, nebulae, galaxie ...
es. These stars contract as they age, increasing core temperature and density to high enough levels to enable the alpha process. Requirements increase with atomic mass, especially in later stages – sometimes referred to as silicon burning – and thus most commonly occur in supernovae
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion. The original ob ...
. Type II supernovae mainly synthesize oxygen and the alpha-elements ( Ne, Mg, Si, S, Ar, Ca, and Ti) while Type Ia supernova
A Type Ia supernova (read: "type one-A") is a type of supernova that occurs in binary systems (two stars orbiting one another) in which one of the stars is a white dwarf. The other star can be anything from a giant star to an even smaller white ...
e mainly produce elements of the iron peak
The iron peak is a local maximum in the vicinity of Iron, Fe (Chromium, Cr, Manganese, Mn, Fe, Cobalt, Co and Nickel, Ni) on the graph of the abundances of the chemical elements.
For elements lighter than iron on the periodic table, nuclear fusio ...
( Ti, V, Cr, Mn, Fe, Co, and Ni). Sufficiently massive stars can synthesize elements up to and including the iron peak solely from the hydrogen and helium that initially comprises the star.
Typically, the first stage of the alpha process (or alpha-capture) follows from the helium-burning stage of the star once helium becomes depleted; at this point, free capture helium to produce . This process continues after the core finishes the helium burning phase as a shell around the core will continue burning helium and convecting into the core. The second stage ( neon burning) starts as helium is freed by the photodisintegration of one atom, allowing another to continue up the alpha ladder. Silicon burning is then later initiated through the photodisintegration of in a similar fashion; after this point, the peak discussed previously is reached. The supernova shock wave produced by stellar collapse provides ideal conditions for these processes to briefly occur.
During this terminal heating involving photodisintegration and rearrangement, nuclear particles are converted to their most stable forms during the supernova and subsequent ejection through, in part, alpha processes. Starting at and above, all the product elements are radioactive and will therefore decay into a more stable isotope; for instance, is formed and decays into .
Special notation for relative abundance
The abundance of total alpha elements in stars is usually expressed in terms oflogarithm
In mathematics, the logarithm of a number is the exponent by which another fixed value, the base, must be raised to produce that number. For example, the logarithm of to base is , because is to the rd power: . More generally, if , the ...
s, with astronomers customarily using a square bracket notation:
:
where is the number of alpha elements per unit volume, and is the number of iron nuclei per unit volume. It is for the purpose of calculating the number that which elements are to be considered "alpha elements" becomes contentious. Theoretical galactic evolution models predict that early in the universe there were more alpha elements relative to iron.
References
Further reading
* {{Star Nuclear fusion Nucleosynthesis