Alpha-thalassemia Trait on:
[Wikipedia]
[Google]
[Amazon]
Alpha-thalassemia (α-thalassemia, α-thalassaemia) is an inherited blood disorder and a form of
 Alpha-thalassemia is almost always inherited. It is a Dominance (genetics), recessive trait - a single defective gene is insufficient to cause illness. Due to the involvement of four alpha globin genes, the inheritance pattern is complex, with varying severity depending on the number of gene mutations inherited from each parent.
Normal individuals carry 4 alpha-globin genes, comprising Autosome, autosomal pairs of the HBA1 and HBA2 genes. There are approximately 130 known mutations which can cause alpha thalassemia, mainly comprising Deletion (genetics), deletion of part or all of a gene which then fails to produce alpha globin. If either one gene or two out of the four is faulty, the remaining genes produce sufficient alpha globin for normal life. If three genes are faulty, the sole functioning gene produces relatively small quantities of alpha globin, causing anemia and Hemoglobin H disease, HbH disease. Four faulty genes (and therefore zero alpha globin) is incompatible with life.
In rare cases alpha thalassemia can be acquired as a consequence of Myelodysplastic syndrome, myelodysplastic cancer.
Alpha-thalassemia is almost always inherited. It is a Dominance (genetics), recessive trait - a single defective gene is insufficient to cause illness. Due to the involvement of four alpha globin genes, the inheritance pattern is complex, with varying severity depending on the number of gene mutations inherited from each parent.
Normal individuals carry 4 alpha-globin genes, comprising Autosome, autosomal pairs of the HBA1 and HBA2 genes. There are approximately 130 known mutations which can cause alpha thalassemia, mainly comprising Deletion (genetics), deletion of part or all of a gene which then fails to produce alpha globin. If either one gene or two out of the four is faulty, the remaining genes produce sufficient alpha globin for normal life. If three genes are faulty, the sole functioning gene produces relatively small quantities of alpha globin, causing anemia and Hemoglobin H disease, HbH disease. Four faulty genes (and therefore zero alpha globin) is incompatible with life.
In rare cases alpha thalassemia can be acquired as a consequence of Myelodysplastic syndrome, myelodysplastic cancer.
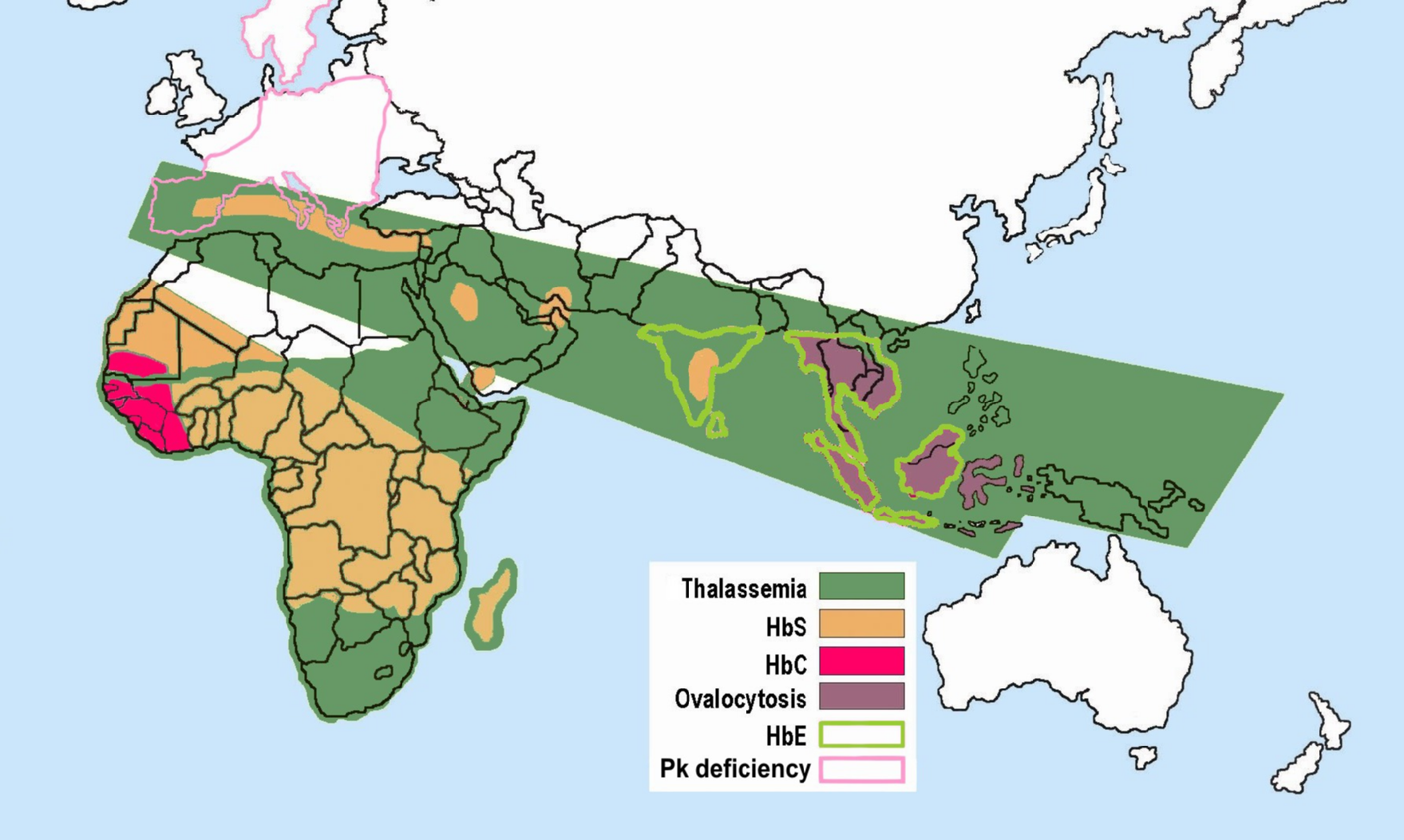 Some hemoglobinopathies seem to have given an evolutionary benefit, especially to heterozygotes, in areas where malaria is Endemic (epidemiology), endemic. Having a mild form of alpha thalassemia has been demonstrated to Human genetic resistance to malaria, protect against malaria and thus can be an advantage in malaria endemic areas, thus conferring a selective survival advantage on carriers (known as heterozygous advantage), and perpetuating the mutation.
Alpha thalassemia genes have a high prevalence in populations originating in sub-Saharan Africa, Mediterranean Sea, Mediterranean, Middle East, and Southeast Asia, southeast and east Asia; all areas which historically have been malaria endemic. The prevalence of these genes has increased in previously non-endemic areas as a consequence of migration flows, slave-trade, and colonization.
A number of mechanisms have been proposed to explain the increased chance of survival for the carrier of an abnormal hemoglobin trait.
Some hemoglobinopathies seem to have given an evolutionary benefit, especially to heterozygotes, in areas where malaria is Endemic (epidemiology), endemic. Having a mild form of alpha thalassemia has been demonstrated to Human genetic resistance to malaria, protect against malaria and thus can be an advantage in malaria endemic areas, thus conferring a selective survival advantage on carriers (known as heterozygous advantage), and perpetuating the mutation.
Alpha thalassemia genes have a high prevalence in populations originating in sub-Saharan Africa, Mediterranean Sea, Mediterranean, Middle East, and Southeast Asia, southeast and east Asia; all areas which historically have been malaria endemic. The prevalence of these genes has increased in previously non-endemic areas as a consequence of migration flows, slave-trade, and colonization.
A number of mechanisms have been proposed to explain the increased chance of survival for the carrier of an abnormal hemoglobin trait.
thalassemia
Thalassemias are a group of Genetic disorder, inherited blood disorders that manifest as the production of reduced hemoglobin. Symptoms depend on the type of thalassemia and can vary from none to severe, including death. Often there is mild to ...
. Thalassemias are a group of inherited blood conditions which result in the impaired production of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
, the molecule that carries oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
in the blood. Symptoms depend on the extent to which hemoglobin is deficient, and include anemia, pallor, Fatigue, tiredness, Splenomegaly, enlargement of the spleen, iron overload, abnormal bone structure, jaundice, and Gallstone, gallstones. In severe cases death ensues, often in infancy, or death of the unborn fetus.
The disease is characterised by reduced production of the Hemoglobin subunit alpha, alpha-globin component of hemoglobin, caused by inherited mutations affecting the genes ''HBA1'' and ''HBA2.'' This causes reduced levels of hemoglobin leading to anemia, while the accumulation of surplus Hemoglobin subunit beta, beta-globin, the other structural component of hemoglobin, damages Red blood cell, red blood cells and shortens their life. Diagnosis is by checking the medical history of near relatives, microscopic examination of blood smear, ferritin test, hemoglobin electrophoresis, and DNA sequencing.
As an inherited condition, alpha thalassemia cannot be prevented although Genetic counseling, genetic counselling of parents prior to Fertilisation, conception can propose the use of Sperm donation, donor sperm or Egg donation, eggs. The principle form of management is blood transfusion every 3 to 4 weeks, which relieves the anemia but leads to iron overload and possible Immune response, immune reaction. Medication includes folate supplementation, Chelation therapy, iron chelation, Bisphosphonate, bisphosphonates, and Splenectomy, removal of the spleen. Alpha thalassemia can also be treated by Hematopoietic stem cell transplantation, bone marrow transplant from a well matched donor.
Thalassemias were first identified in severely sick children in 1925, with identification of alpha and beta subtypes in 1965. Alpha thalassemia has its greatest prevalence in populations originating from Southeast Asia, Mediterranean countries, Africa, the Middle East, India, and Central Asia. Having a mild form of alpha thalassemia has been demonstrated to Human genetic resistance to malaria, protect against malaria and thus can be an advantage in malaria Endemic (epidemiology), endemic areas.
Cause
 Alpha-thalassemia is almost always inherited. It is a Dominance (genetics), recessive trait - a single defective gene is insufficient to cause illness. Due to the involvement of four alpha globin genes, the inheritance pattern is complex, with varying severity depending on the number of gene mutations inherited from each parent.
Normal individuals carry 4 alpha-globin genes, comprising Autosome, autosomal pairs of the HBA1 and HBA2 genes. There are approximately 130 known mutations which can cause alpha thalassemia, mainly comprising Deletion (genetics), deletion of part or all of a gene which then fails to produce alpha globin. If either one gene or two out of the four is faulty, the remaining genes produce sufficient alpha globin for normal life. If three genes are faulty, the sole functioning gene produces relatively small quantities of alpha globin, causing anemia and Hemoglobin H disease, HbH disease. Four faulty genes (and therefore zero alpha globin) is incompatible with life.
In rare cases alpha thalassemia can be acquired as a consequence of Myelodysplastic syndrome, myelodysplastic cancer.
Alpha-thalassemia is almost always inherited. It is a Dominance (genetics), recessive trait - a single defective gene is insufficient to cause illness. Due to the involvement of four alpha globin genes, the inheritance pattern is complex, with varying severity depending on the number of gene mutations inherited from each parent.
Normal individuals carry 4 alpha-globin genes, comprising Autosome, autosomal pairs of the HBA1 and HBA2 genes. There are approximately 130 known mutations which can cause alpha thalassemia, mainly comprising Deletion (genetics), deletion of part or all of a gene which then fails to produce alpha globin. If either one gene or two out of the four is faulty, the remaining genes produce sufficient alpha globin for normal life. If three genes are faulty, the sole functioning gene produces relatively small quantities of alpha globin, causing anemia and Hemoglobin H disease, HbH disease. Four faulty genes (and therefore zero alpha globin) is incompatible with life.
In rare cases alpha thalassemia can be acquired as a consequence of Myelodysplastic syndrome, myelodysplastic cancer.
Pathophysiology
Symptoms
Symptoms depend on the type and severity of thalassemia. Hereditary carrier, Carriers of thalassemia genes may have no symptoms or very mild symptoms with occasional crisis; those with three or more (out of four) affected genes will have severe and life threatening symptoms. Full alpha thalassemia with all four genes failing to synthesise alpha-globin, is generally fatal to the unborn child. The absence of alpha globin means that zero functional hemoglobin is produced during gestation. Unmatched gamma globin chains cluster to form Hemoglobin Barts, hemoglobin Bart's, which is ineffective at transporting oxygen. In this situation, a fetus will develop hydrops fetalis, a form of edema, which can be detected on prenatal ultrasound. The child will normally die before or shortly after birth, unless intrauterine blood transfusion is performed. Less severe alpha thalassemia may affect growth and development. If thalassemia is untreated or undetected in the infant, this can lead to developmental issues such as slowed growth, delayed puberty, bone abnormalities, and intellectual impairment. More generally, impaired production of hemoglobin causes Anemia of chronic disease, anemia, resulting in tiredness and a general lack of energy, shortness of breath, rapid or irregular heartbeat, dizziness, pale skin, yellowing of the skin and eyes (jaundice). In thalassemia, ineffective erythropoiesis causes the bone marrow to expand. This expansion is a compensatory response to the damage caused to red blood cells by the imbalanced production of globin chains. Bone marrow expansion can lead to abnormal bone structure, particularly in the skull and face. Expansion of the bone marrow in the developing child leads to a distinctive facial shape often referred to as "Chipmunk Facies (medical), facies". Other skeletal changes include osteoporosis, Delayed milestone, growth retardation, and Kyphosis, malformation of the spine. People with thalassemia can get Iron overload, too much iron in their bodies, either from the disease itself as RBCs are destroyed, or as a consequence of frequent blood transfusions. Excess iron is not excreted, but forms toxic Iron preparation#Iron toxicity and treatment, non-transferrin-bound iron. This can lead to organ damage, potentially affecting the heart, liver, endocrine system, bones and spleen. Symptoms include an irregular heartbeat, cardiomyopathy, cirrhosis of the liver, hypothyroidism, delayed puberty and fertility problems, brittle and deformed bones, and an enlarged spleen. The spleen is the organ which removes damaged red blood cells from circulation; in thalassemia patients it is abnormally active, causing it to Splenomegaly, enlarge and possibly become hyperactive, a condition called Splenomegaly, hypersplenism. The immune system can become compromised in a number of ways; anemia, iron overload, and hypersplenism may affect the immune response and increase the risk of severe infection.Diagnosis
Prognosis
The prognosis for alpha thalassemia depends on the degree to which alpha globin production is affected. Those with mild alpha thalassemia, involving deletion of one or two alpha-globin genes, do not generally require treatment and have a normal life expectancy. Hemoglobin H disease, with three of the four genes either deleted or inactive, gives a mild to moderate form of anemia but may lead normal lives. The prognosis when all four genes are affected, leading to Hb Bart's hydrops fetalis, is very poor, with most affected fetuses dying ''wikt:in_utero, in utero'' or shortly after birth due to severe Intrauterine hypoxia, fetal hypoxia. It can be treated with Intrauterine transfusion, intrauterine transfusions, however survival remains low and the infant requires lifelong blood transfusions. As of 2017, 69 patients were known who have survived past infancy.Treatment
Treatment for thalassemia depends on the severity of the disease. People with thalassemia Phenotypic trait, traits (thalassemia minor or non transfusion dependent thalassemia), may not require medical or follow-up care after the initial diagnosis is made. Occasionally transfusions may be necessary particularly around childbirth, surgery, or if other conditions provoke anemia. A folic acid supplement may also be recommended. For those with severe forms of thalassemia (thalassemia major, or transfusion-dependent thalassemia), the three principal treatments are red blood cell transfusions to relieve anemia, iron chelation to mitigate the side effects of transfusion, and folic acid supplementation to encourage the growth of new blood cells. Other forms of treatment available depending on individual circumstances.Red blood cell transfusions
Blood transfusion, Blood transfusions are the main treatment approach for prolonging life. Donated healthy red blood cells have a functional life of 4 to 6 weeks before they wear out and are broken down in the spleen. Regular transfusions every three to four weeks are necessary in order to maintain hemoglobin at a healthy level. Transfusions come with risks including iron overload, the risk of acquiring infections, and the risk of immune reaction to the donated cells (Alloimmunity, alloimmunization).Iron chelation
Multiple blood transfusions lead to severe iron overload, as the body eventually breaks down the hemoglobin in donated cells. This releases iron which it is unable to excrete. Iron overload may be treated by chelation therapy with the medications deferoxamine, deferiprone, or deferasirox. Deferoxamine is only effective as a daily injection, complicating its long-term use. Adverse effects include primary skin reactions around the injection site and hearing loss. Deferasirox and deferiprone are both oral medications, whose common side effects include nausea, vomiting and diarrhea.Folic acid
Folate is a B group vitamin which is involved in the manufacture of red blood cells. Folate supplementation, in the form of folic acid, is often recommended in thalassemia.Osteoporosis
People with thalassemia are at a higher risk of osteoporosis. Treatment options include Bisphosphonate, bisphosphonates and Zinc, zinc supplementation.Removal of the spleen
The spleen is the organ which removes damaged or misshapen red blood cells from the circulation. In thalassemia, this can lead to the spleen becoming enlarged, a condition known as splenomegaly. Slight enlargement of the spleen is not a problem, however if it becomes extreme then surgical removal of the spleen (splenectomy) may be recommended.Hematopoietic stem cell transplantation
Hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for both alpha and beta thalassemia. It involves replacing the dysfunctional Stem cell, stem cells in the bone marrow with healthy cells from a well-matched Organ donation, donor. Cells are ideally sourced from human leukocyte antigen matched relatives; the procedure is more likely to succeed in children rather than adults. The first HSC transplant for thalassemia was carried out in 1981 on a patient with beta thalassemia major. Since then, a number of patients have received bone marrow transplants from healthy matched donors, although this procedure has a high level of risk. In 2018 an unborn child with hydrops fetalis, a potentially fatal complication of alpha thalassemia, was successfully transfused ''wikt:in_utero, in utero'' with her mother's stem cells. HSCT is a dangerous procedure with many possible complications; it is reserved for patients with life-threatening diseases. Risks associated with HSCT can include Graft-versus-host disease, graft-versus host disease, Transplant rejection, failure of the graft, and other toxicity related to the transplant. In one study of 31 people, the procedure was successful for 22 whose hemoglobin levels improved to the normal range, in seven the graft failed and they continued to live with thalassemia, and two died of transplantation-related causes.Evolutionary advantage
 Some hemoglobinopathies seem to have given an evolutionary benefit, especially to heterozygotes, in areas where malaria is Endemic (epidemiology), endemic. Having a mild form of alpha thalassemia has been demonstrated to Human genetic resistance to malaria, protect against malaria and thus can be an advantage in malaria endemic areas, thus conferring a selective survival advantage on carriers (known as heterozygous advantage), and perpetuating the mutation.
Alpha thalassemia genes have a high prevalence in populations originating in sub-Saharan Africa, Mediterranean Sea, Mediterranean, Middle East, and Southeast Asia, southeast and east Asia; all areas which historically have been malaria endemic. The prevalence of these genes has increased in previously non-endemic areas as a consequence of migration flows, slave-trade, and colonization.
A number of mechanisms have been proposed to explain the increased chance of survival for the carrier of an abnormal hemoglobin trait.
Some hemoglobinopathies seem to have given an evolutionary benefit, especially to heterozygotes, in areas where malaria is Endemic (epidemiology), endemic. Having a mild form of alpha thalassemia has been demonstrated to Human genetic resistance to malaria, protect against malaria and thus can be an advantage in malaria endemic areas, thus conferring a selective survival advantage on carriers (known as heterozygous advantage), and perpetuating the mutation.
Alpha thalassemia genes have a high prevalence in populations originating in sub-Saharan Africa, Mediterranean Sea, Mediterranean, Middle East, and Southeast Asia, southeast and east Asia; all areas which historically have been malaria endemic. The prevalence of these genes has increased in previously non-endemic areas as a consequence of migration flows, slave-trade, and colonization.
A number of mechanisms have been proposed to explain the increased chance of survival for the carrier of an abnormal hemoglobin trait.
Combination hemoglobinopathies
A combination hemoglobinopathy occurs when someone inherits two different abnormal hemoglobin genes. Alpha thalassemia can coexist with other hemoglobinopathies such as sickle cell disease and beta thalassemia. When alpha thalassemia carrier or trait combines with another hemoglobinopathy, the symptoms are generally those of the other .See also
* Thalassemia * Beta-thalassemia * Delta-thalassemia * HemoglobinopathyReferences
Further reading
* *External links
* {{DEFAULTSORT:Alpha-Thalassemia Disorders of globin and globulin proteins Hereditary hemolytic anemias