Alpha-neurotoxin on:
[Wikipedia]
[Google]
[Amazon]
 α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause
α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause
 α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause
α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis
Paralysis (also known as plegia) is a loss of motor function in one or more muscles. Paralysis can also be accompanied by a loss of feeling (sensory loss) in the affected area if there is sensory damage. In the United States, roughly 1 in 50 ...
, respiratory failure, and death. Members of the three-finger toxin protein family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be c ...
, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse
In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or to the target effector cell.
Synapses are essential to the transmission of nervous impulses from ...
that bind competitively and irreversibly, preventing synaptic acetylcholine
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals (including humans) as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Part ...
(ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.
History
The term α-neurotoxin was coined byC.C. Chang
CC, cc, or C-C may refer to:
Arts, entertainment, and media Fictional characters
* C.C. (''Code Geass''), a character in the ''Code Geass'' anime series, pronounced "C-two"
* C.C. Babcock, a character in the American sitcom ''The Nanny''
* Come ...
, who designated the postsynaptic bungarotoxin with the α- prefix because it happened to be slowest moving of the bungarotoxins under starch zone electrophoresis. The "α-" prefix subsequently came to connote any toxins with postsynaptic action. Members of this group are sometimes referred to as "curaremimetics" due to the similarity of their effects with the plant alkaloid curare.
As more snake venoms were characterized, many were found to contain homologous nAChR-antagonist proteins. These came to be collectively known as the snake venom α-neurotoxins.
General structure
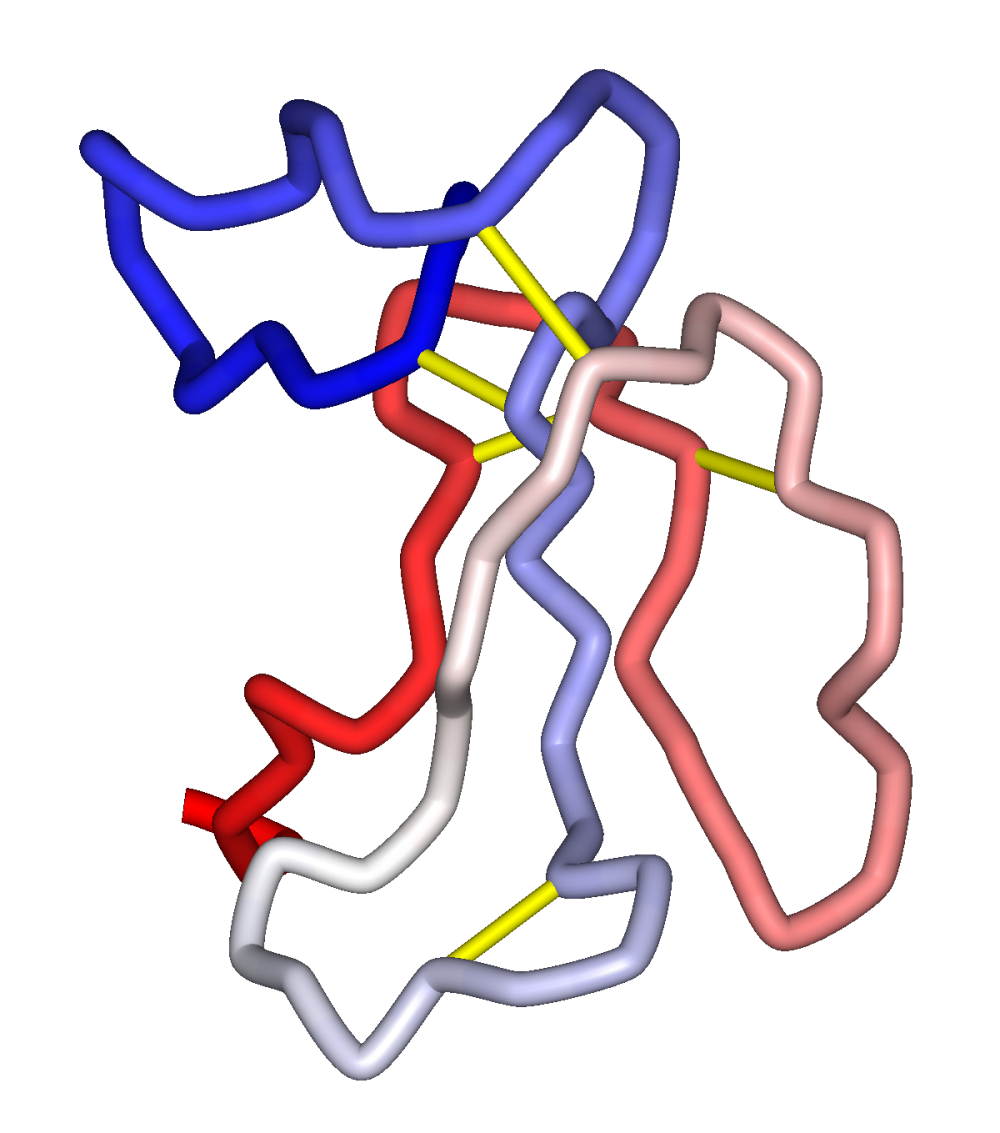
All α-neurotoxins share the three-finger toxin tertiary structure, consisting of a small globular core containing four disulfide bonds, three loops or "fingers", and a C-terminal tail. The class can be divided into two groups distinguished by length; short-chain neurotoxins have 60-62 residues and only the four core disulfide bonds characteristic of the fold, while long-chain neurotoxins have 66 or more residues, often including a longerC-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
, and an additional disulfide bond in the second "finger" loop. These classes have significant sequence homology and share the same three-dimensional structure, but have differing specificities and kinetics of association/dissociation with the receptor. Localized mobility at the tips of fingers I and II is essential for binding. Accordingly, mutation of these residues produces large effects on binding. The additional disulfide bond in the second loop of the long-chain forms is likewise thought to influence binding specificity. Although both short and long-chain neurotoxins bind the same site on their target receptors, short-chain neurotoxins do not potently block α7 homo-oligomeric neuronal AChRs, while long-chain neurotoxins do. α-bungarotoxin and α-cobratoxin are both long-type.
Functions
''For specifics, see Alpha-Bungarotoxin and nicotinic acetylcholine receptor'' α-Neurotoxins antagonistically bind tightly and noncovalently to nAChRs of skeletal muscles, thereby blocking the action of ACh at the postsynaptic membrane, inhibiting ion flow and leading to paralysis. nAChRs contain two binding sites for snake venom neurotoxins. Some computational studies of the mechanism of inhibition usingnormal mode
A normal mode of a dynamical system is a pattern of motion in which all parts of the system move sinusoidally with the same frequency and with a fixed phase relation. The free motion described by the normal modes takes place at fixed frequencies. ...
dynamics suggest that a twist-like motion caused by ACh binding may be responsible for pore opening, and that this motion is inhibited by toxin binding.
Evolution
Although three-finger protein domains are widespread, three-finger toxins appear only in snakes, and are particularly enriched in elapids. There is evidence that alpha-neurotoxins have evolved rapidly and are subject topositive selection
In population genetics, directional selection, is a mode of negative natural selection in which an extreme phenotype is favored over other phenotypes, causing the allele frequency to shift over time in the direction of that phenotype. Under dir ...
, possibly due to an evolutionary arms race with prey species.
Snake nAchRs have specific sequence features that render them poor binding partners for alpha-neurotoxins. Some mammal
Mammals () are a group of vertebrate animals constituting the class Mammalia (), characterized by the presence of mammary glands which in females produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or ...
ian lineages also display mutations conferring resistance to alpha-neurotoxins; such resistance is believed to have evolved convergently at least four times in mammals, reflecting two different biochemical mechanisms of adaptation. The introduction of glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
sites on the receptor, resulting in steric hindrance at the neurotoxin binding site, is a well-characterized resistance mechanism found in mongooses, while the honey badger, domestic pig, and hedgehog lineages replace aromatic amino acids with charged residues; at least in some lineages, these molecular adaptations likely reflect predation on venomous snakes.
References
{{Nicotinic acetylcholine receptor modulators Neurotoxins Nicotinic antagonists Venomous snakes