Aldehyde Ferredoxin Oxidoreductase on:
[Wikipedia]
[Google]
[Amazon]
In
 Two
Two
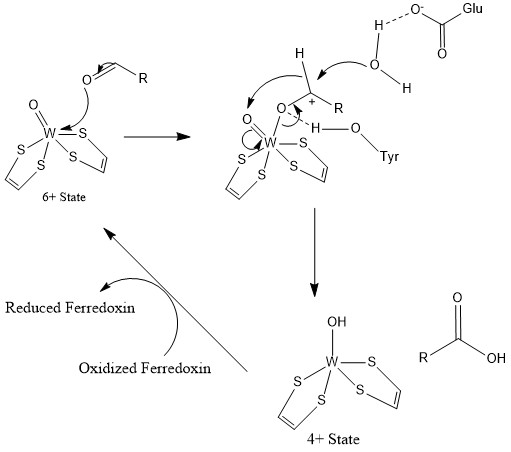 General Reaction Mechanism of AOR:
:RCHO + H2O → RCO2H + 2H+ + 2 e−
The redox equivalents are provided by the 4Fe-4S cluster.
A tyrosine residue is proposed to activate the electrophilic centre of aldehydes by H-bonding to the carbonyl oxygen atom, coordinated to the W centre. A glutamic acid residue near the active site activates a water molecule for a nucleophilic attack on aldehyde carbonyl center. After nucleophilic attack by water, hydride is transferred to oxo-tungsten sie thus, . Subsequently, W(VI) is regenerated by electron transfer to the 4Fe-4S center. With formaldehyde ferredoxin oxidoreductase, Glu308 and Tyr 416 would be involved while Glu313 and His448 is shown to be present in AOR active site.
General Reaction Mechanism of AOR:
:RCHO + H2O → RCO2H + 2H+ + 2 e−
The redox equivalents are provided by the 4Fe-4S cluster.
A tyrosine residue is proposed to activate the electrophilic centre of aldehydes by H-bonding to the carbonyl oxygen atom, coordinated to the W centre. A glutamic acid residue near the active site activates a water molecule for a nucleophilic attack on aldehyde carbonyl center. After nucleophilic attack by water, hydride is transferred to oxo-tungsten sie thus, . Subsequently, W(VI) is regenerated by electron transfer to the 4Fe-4S center. With formaldehyde ferredoxin oxidoreductase, Glu308 and Tyr 416 would be involved while Glu313 and His448 is shown to be present in AOR active site.
enzymology
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
, an aldehyde ferredoxin oxidoreductase () is an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
that catalyzes
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
the chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
:an aldehyde + H2O + 2 oxidized ferredoxin an acid + 3 H+ + 2 reduced ferredoxin
This enzyme belongs to the family of oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually ut ...
s, specifically those acting on the aldehyde or oxo group of donor with an iron-sulfur protein as acceptor. The systematic name
A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivi ...
of this enzyme class is aldehyde:ferredoxin oxidoreductase. This enzyme is also called AOR. It is a relatively rare example of a tungsten-containing protein.
Occurrence
The active site of the AOR family feature an oxo-tungsten center bound to a pair ofmolybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopte ...
cofactors (which does not contain molybdenum) and an 4Fe-4S cluster. This family includes AOR, formaldehyde ferredoxin oxidoreductase (FOR), glyceraldehyde-3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms.Nelson, D ...
ferredoxin oxidoreductase (GAPOR), all isolated from hyperthermophilic archea
Archaea ( ) is a domain of organisms. Traditionally, Archaea only included its prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even though the domain Archaea cladis ...
; carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
reductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually uti ...
found in clostridia; and hydroxycarboxylate viologen oxidoreductase from ''Proteus vulgaris'', the sole member of the AOR family containing molybdenum. GAPOR may be involved in glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
, but the functions of the other protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s are not yet clear. AOR has been proposed to be the primary enzyme responsible for oxidising the aldehydes that are produced by the 2-keto acid oxidoreductases
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually ut ...
.
AOR is found in hyperthermophillic archaea, Pyrococcus furiosus
''Pyrococcus furiosus'' is a heterotrophic, strictly anaerobic, extremophilic, model species of archaea. It is classified as a hyperthermophile because it thrives best under extremely high temperatures, and is notable for having an optimum gr ...
. The archaeons ''Pyrococcus'' ES-4 strain and ''Thermococcus'' ES-1 strain differ by their substrate specificity: AFOs show a broader size range of its aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
substrates. Its primary role is to oxidize aldehyde coming derived from the metabolism of amino acids and glucoses. Aldehyde Ferredoxin Oxidoreductase is a member of an AOR family, which includes glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) and Formaldehyde Ferredoxin Oxidoreductase.
Function
AOR functions at high temperature conditions (~80 degrees Celsius) at an optimal pH of 8-9. It is oxygen-sensitive as it loses bulk of its activity from oxygen exposure and works in the cytoplasm where it is a reducing environment. Thus, either exposure to oxygen or lowering of the temperature causes an irreversible loss of its catalytic properties. Also, as a result of oxygen sensitivity of AOR, purification of the enzyme is done underanoxic
Anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of dissolved ox ...
environments.
It is proposed that AOR has a role in the Entner-Doudoroff pathway (glucose degradation) due to its increased activity with maltose
}
Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the tw ...
incorporation. However, other proposals include its role in oxidation of amino acid metabolism aldehyde side products coming from de-aminated 2-ketoacids. The main substrates for aldehyde ferredoxin oxidoreductase are acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most ...
, phenylacetaldehyde
Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compound ...
, and isovalerdehyde, which is a metabolic product from common amino acids and glucose. For example, acetaldehyde reaches its kcat/KM value up to 22.0 μM-1s-1. In fact, some microorganisms only make use of amino acids as a carbon source, such as Thermococcus strain ES1; thus, they utilize aldehyde ferredoxin oxidoreductase to metabolize the amino acid carbon source.
Structure
AOR is homodimeric. Each 67kDa subunit contains 1 tungsten and 4-5Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
atoms. The two subunits are bridged by a low spin Iron center. It is believed that the two subunits function independently.
;Tungsten-pterin
Tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
in the active site of AOR adopts a distorted square pyramidal geometry bound an oxo/hydroxo ligand and the dithiolene
Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two dono ...
substituents of two molybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopte ...
cofactors.
molybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopte ...
cofactors bind tungsten, as observed in many related enzymes. Tungsten is not bonded directly to the protein. Phosphate centers pendant on the cofactor are bound to a Mg2+, which is also bound by Asn93 and Ala183 to complete its octahedral coordination sphere. Thus, pterin and Tungsten atoms are connected to the AOR enzyme primarily through pterin's Hydrogen bonding networks with the amino acid residues. In addition, two water ligands that occupy the octahedral geometry take part in hydrogen bonding networks with pterin, phosphate, and Mg2+. While e4S4cluster is bound by four Cys ligands, Pterin - rich in amino and ether linkages - interacts with the Asp-X-X-Gly-Leu-(Cys/Asp) sequences in the AOR enzyme. In such sequence, Cys494 residue is also hydrogen bonded to the e4S4cluster. This indicates that Cys494 residue connects the Tungsten site and the e4S4cluster site in the enzyme. Iron atom in the cluster is additionally bound by three other Cystein ligands: . Also, another linker amino acid residue between ferredoxin cluster and pterin is the Arg76, which hydrogen bonds to both pterin and ferredoxin. It is proposed that such hydrogen bonding interactions imply pterin cyclic ring system as an electron carrier. Additionally the C=''O'' center of the pterin binds Na+. The W=O center is proposed, not verified crystallographically.
AOR consists of three domains, domain 1, 2, and 3. While domain 1 contains pterin bound to tungsten, the other two domains provide a channel from tungsten to protein's surface (15 Angstroms in length) in order to allow specific substrates to enter the enzyme through its channel. In the active site, this pterin molecules is in a saddle-like conformation (500 to the normal plane) to “sit” on the domain 1 which also takes on a form with beta sheets to accommodate the Tungsten-Pterin site.
;Iron
The iron center in between the two subunits serve a structural role in AOR. Iron metal atoms takes on a tetrahedral conformation while the ligand coordination comes from two histidines and glutamic acids. This is not known to have any functional role in the redox activity of the protein.
;Fe4S4 centre
e4S4cluster in AOR is different in some aspects to other ferredoxin molecules. EPR measurements confirm that it serves as a one-electron shuttle.
Aldehyde ferredoxin oxidoreductase mechanism
In the catalytic cycle, W(VI) (tungsten "six") converts to W(IV) concomitant with oxidation of the aldehyde to a carboxylic acid (equivalently, a carboxylate). A W(V) intermediate can be detected byEPR spectroscopy
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
.
 General Reaction Mechanism of AOR:
:RCHO + H2O → RCO2H + 2H+ + 2 e−
The redox equivalents are provided by the 4Fe-4S cluster.
A tyrosine residue is proposed to activate the electrophilic centre of aldehydes by H-bonding to the carbonyl oxygen atom, coordinated to the W centre. A glutamic acid residue near the active site activates a water molecule for a nucleophilic attack on aldehyde carbonyl center. After nucleophilic attack by water, hydride is transferred to oxo-tungsten sie thus, . Subsequently, W(VI) is regenerated by electron transfer to the 4Fe-4S center. With formaldehyde ferredoxin oxidoreductase, Glu308 and Tyr 416 would be involved while Glu313 and His448 is shown to be present in AOR active site.
General Reaction Mechanism of AOR:
:RCHO + H2O → RCO2H + 2H+ + 2 e−
The redox equivalents are provided by the 4Fe-4S cluster.
A tyrosine residue is proposed to activate the electrophilic centre of aldehydes by H-bonding to the carbonyl oxygen atom, coordinated to the W centre. A glutamic acid residue near the active site activates a water molecule for a nucleophilic attack on aldehyde carbonyl center. After nucleophilic attack by water, hydride is transferred to oxo-tungsten sie thus, . Subsequently, W(VI) is regenerated by electron transfer to the 4Fe-4S center. With formaldehyde ferredoxin oxidoreductase, Glu308 and Tyr 416 would be involved while Glu313 and His448 is shown to be present in AOR active site.
References
Further reading
* * * {{InterPro content, IPR013983 EC 1.2.7 Enzymes of unknown structure