Acrosin on:
[Wikipedia]
[Google]
[Amazon]
Acrosin is a digestive
 The reaction proceeds according to the usual
The reaction proceeds according to the usual
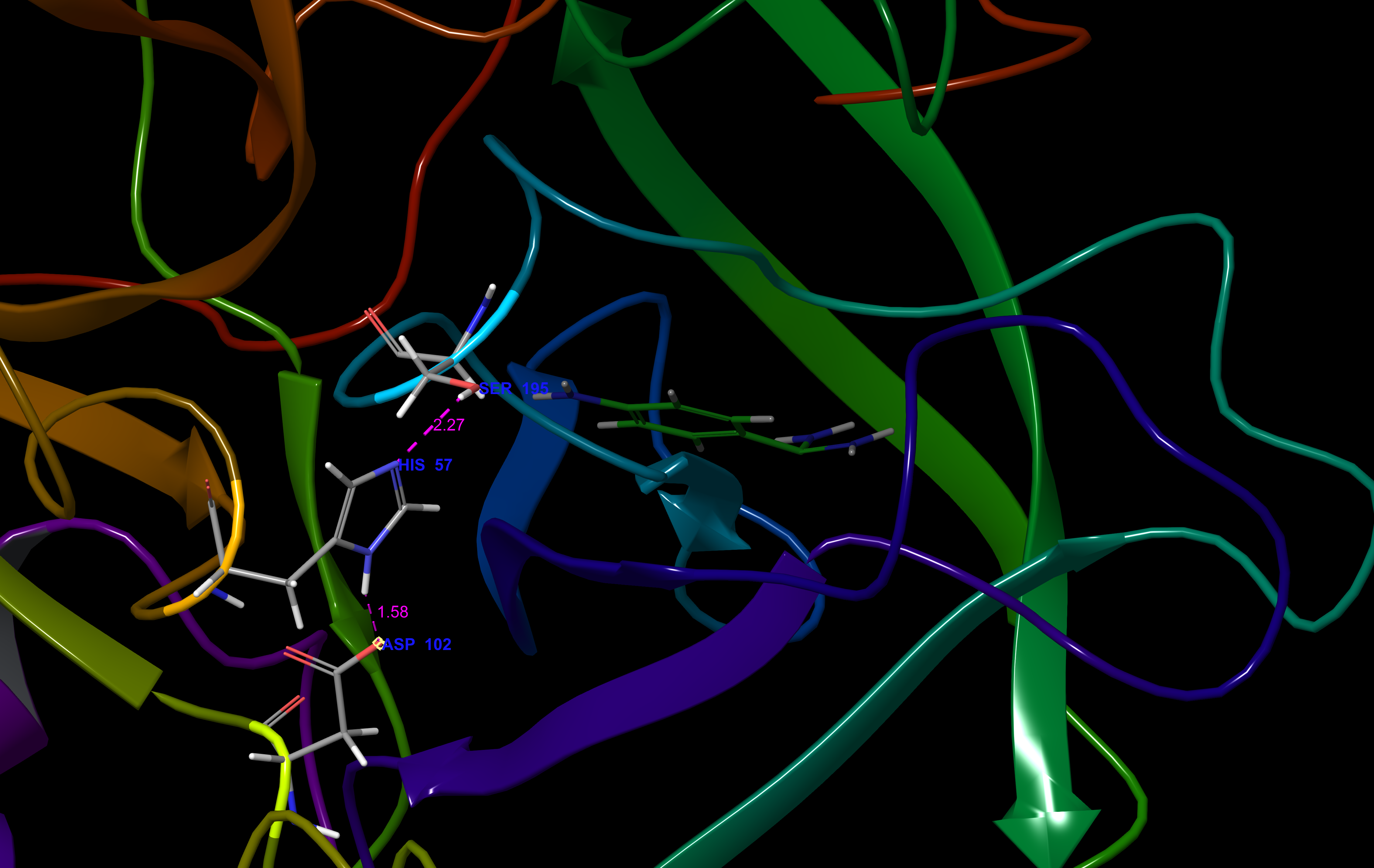
 The catalytic triad consists of residues His-57, Asp-102, and Ser-195. These residues are found in a binding pocket that has been termed the "S1" pocket, consistent with the naming scheme that has been adopted for other proteases. The S1 pocket regulates acrosin's specificity for Arg and Lys substrates, with a conserved Trp-215 serving as a "gatekeeper" residue for the binding site entrance.
The catalytic triad consists of residues His-57, Asp-102, and Ser-195. These residues are found in a binding pocket that has been termed the "S1" pocket, consistent with the naming scheme that has been adopted for other proteases. The S1 pocket regulates acrosin's specificity for Arg and Lys substrates, with a conserved Trp-215 serving as a "gatekeeper" residue for the binding site entrance.
 An important structural element of β-acrosin is a highly charged patch (formed through both amino acids and post-translational modifications) on its surface region, that has been termed the "anion binding exosite." This site consists of an area of excess positive charge, which has been hypothesized to be important in binding to the matrix of the zona pellucida, a heavily glycosylated and sulfated region with excess negative charge. This structural feature is consistent with the secondary binding protein hypothesis, as charge-charge interactions would stabilize a protein-zona pellucida "tethering" complex. Further consistent with this structural hypothesis is the knowledge that
An important structural element of β-acrosin is a highly charged patch (formed through both amino acids and post-translational modifications) on its surface region, that has been termed the "anion binding exosite." This site consists of an area of excess positive charge, which has been hypothesized to be important in binding to the matrix of the zona pellucida, a heavily glycosylated and sulfated region with excess negative charge. This structural feature is consistent with the secondary binding protein hypothesis, as charge-charge interactions would stabilize a protein-zona pellucida "tethering" complex. Further consistent with this structural hypothesis is the knowledge that
S01.223
* {{Portal bar, Biology, border=no EC 3.4.21
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
that acts as a protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products ...
. In humans, acrosin is encoded by the ''ACR'' gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
. Acrosin is released from the acrosome
The acrosome is an organelle that develops over the anterior (front) half of the head in the spermatozoa (sperm cells) of humans and many other animals. It is a cap-like structure derived from the Golgi apparatus. In placental mammals, the acroso ...
of spermatozoa
A spermatozoon (; also spelled spermatozoön; : spermatozoa; ) is a motile sperm cell (biology), cell produced by male animals relying on internal fertilization. A spermatozoon is a moving form of the ploidy, haploid cell (biology), cell that is ...
as a consequence of the acrosome reaction
For fertilization to happen between a sperm and egg cell, a sperm must first fuse with the plasma membrane and then penetrate the female egg cell to fertilize it. While the fusion of the sperm cell with the egg cell's plasma membrane is relatively ...
. It aids in the penetration of the Zona Pellucida The ''zona pellucida'' (Latin meaning "transparent zone") is the specialized area surrounding mammalian oocytes (eggs). It is also known as an egg coat. The ''zona pellucida'' is essential for oocyte growth and fertilization.
The ''zona pelluc ...
.
Enzyme Mechanism
Acrosin is a typical serine proteinase with trypsin-like specificity. The reaction proceeds according to the usual
The reaction proceeds according to the usual serine protease
Serine proteases (or serine endopeptidases) are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site.
They are found ubiquitously in both eukaryotes and prokaryotes. Serin ...
mechanism. First, His-57 deprotonates Ser-195, allowing it to serve as a nucleophile. Deprotonated Ser-195 then reacts with the carbonyl carbon of a peptide, forming a tetrahedral intermediate. The tetrahedral intermediate then collapses, resulting in an H2N-R1 leaving group, which is protonated through His-57. Finally, His-57 deprotonates a water molecule, which can then serve as a nucleophile by similarly reacting with the carbonyl carbon. Collapse of the tetrahedral intermediate then results in a Ser-195 leaving group, which is protonated through His-57, resulting in all residues returned to their pre-catalytic state, and a carboxylic acid where there was previously a peptide bond.
Biological Function
Acrosin is the major proteinase present in the acrosome of mature spermatozoa. It is stored in the acrosome in its precursor form, proacrosin. Upon stimulus, the acrosome releases its contents onto the zona pellucida. After this reaction occurs, thezymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the activ ...
form of the protease is then processed into its active form, β-acrosin. The active enzyme then functions in the lysis of the zona pellucida, thus facilitating penetration of the sperm through the innermost glycoprotein
Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known a ...
layers of the ovum
The egg cell or ovum (: ova) is the female reproductive cell, or gamete, in most anisogamous organisms (organisms that reproduce sexually with a larger, female gamete and a smaller, male one). The term is used when the female gamete is not capa ...
.
The importance of acrosin in the acrosome reaction has been contested. It has been found through genetic knockout experiments that mouse spermatozoa lacking β-acrosin (the active protease) still have the ability to penetrate the zona pellucida. Thus, some argue for its role in assisting in the dispersal of acrosomal contents following the acrosome reaction, while others demonstrate evidence for its role as a secondary binding protein between the spermatozoa and zona pellucida. Under the secondary binding protein hypothesis, acrosin could serve a role in binding to molecules on the zona pellucida, tethering the spermatozoa to the egg. This "tethering" would ensure penetration due to the applied motile force of the spermatozoa.
Acrosin regulation has been found to occur through protein C inhibitor
Protein C inhibitor (PCI, SERPINA5) is a serine protease inhibitor ( serpin) that limits the activity of protein C (an anticoagulant).
An N-terminal fragment of PCI is a possible serum biomarker for prostate cancer.
Protein C inhibitor is act ...
(PCI). PCI is present in the male reproductive tract at 40x higher concentrations than in blood plasma. PCI has been demonstrated to inhibit the proteolytic activity of acrosin. Thus, PCI has been hypothesized to have a protective role: if acrosomal enzymes were released prematurely, or if the spermatozoa was degenerated within the male reproductive tract, the high concentrations of PCI would inhibit acrosin from inflicting proteolytic damage on nearby tissues.
Structure
β-acrosin demonstrates a high degree of sequence identity (70-80%) between boar, bull, rat, guinea pig, mouse, and human isoforms. There exists a somewhat similar (27-35%) sequence identity between β-acrosin and other serine proteases such astrypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the dig ...
and chymotrypsin
Chymotrypsin (, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenu ...
. While most serine protease
Serine proteases (or serine endopeptidases) are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site.
They are found ubiquitously in both eukaryotes and prokaryotes. Serin ...
s are activated through one cleavage event, proacrosin requires processing at both the N and C-terminal domains. Proacrosin is first cleaved between Arg-22 and adjacent Valine to create a 22 residue light chain, and an active protease termed α-acrosin. This light chain remains associated with the heavy chain, cross-linked through two disulfide bonds
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
In in ...
to form a heterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ...
. Following these N-terminal cleavage events, three cleavages at the C-terminal domain removes 70 residues, yielding β-acrosin. Acrosin has two sites which have been identified as possible N-glycosylation
''N''-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), i ...
sites: Asn-2 and Asn-169. The catalytic triad consists of residues His-57, Asp-102, and Ser-195. These residues are found in a binding pocket that has been termed the "S1" pocket, consistent with the naming scheme that has been adopted for other proteases. The S1 pocket regulates acrosin's specificity for Arg and Lys substrates, with a conserved Trp-215 serving as a "gatekeeper" residue for the binding site entrance.
The catalytic triad consists of residues His-57, Asp-102, and Ser-195. These residues are found in a binding pocket that has been termed the "S1" pocket, consistent with the naming scheme that has been adopted for other proteases. The S1 pocket regulates acrosin's specificity for Arg and Lys substrates, with a conserved Trp-215 serving as a "gatekeeper" residue for the binding site entrance.
 An important structural element of β-acrosin is a highly charged patch (formed through both amino acids and post-translational modifications) on its surface region, that has been termed the "anion binding exosite." This site consists of an area of excess positive charge, which has been hypothesized to be important in binding to the matrix of the zona pellucida, a heavily glycosylated and sulfated region with excess negative charge. This structural feature is consistent with the secondary binding protein hypothesis, as charge-charge interactions would stabilize a protein-zona pellucida "tethering" complex. Further consistent with this structural hypothesis is the knowledge that
An important structural element of β-acrosin is a highly charged patch (formed through both amino acids and post-translational modifications) on its surface region, that has been termed the "anion binding exosite." This site consists of an area of excess positive charge, which has been hypothesized to be important in binding to the matrix of the zona pellucida, a heavily glycosylated and sulfated region with excess negative charge. This structural feature is consistent with the secondary binding protein hypothesis, as charge-charge interactions would stabilize a protein-zona pellucida "tethering" complex. Further consistent with this structural hypothesis is the knowledge that suramin
Suramin is a medication used to treat African sleeping sickness and river blindness. It is the treatment of choice for sleeping sickness without central nervous system involvement. It is given by injection into a vein.
Suramin causes a fai ...
- a polysulfated drug (with substantial corresponding negative charge) has been found to inhibit sperm-zona pellucida binding.
Disease and Pharmaceutical Relevance
While one study which utilized mice models indicated that acrosin is not a necessary component of zona pellucida penetration, other studies in humans have shown an association between low acrosomal proteinase activity and infertility. Other research groups have demonstrated a significant correlation between acrosin activity and sperm motility. In rabbit models, an intravaginal contraceptive device that secreted tetradecyl sodium sulfate, a known inhibitor of acrosin andhyaluronidase
Hyaluronidases are a family of enzymes that catalyse the degradation of hyaluronic acid. Karl Meyer classified these enzymes in 1971, into three distinct groups, a scheme based on the enzyme reaction products. The three main types of hyaluroni ...
s, had a complete contraceptive effect.Burck P.J., Zimmerman R.E. An intravaginal contraceptive device for the delivery of an acrosin and hyaluronidase inhibitor" ''Fertil Steril'' 1984 Feb;41(2):314-8. Although its exact mechanism of action is not entirely clear, acrosin could thus serve as a novel target for contraceptive agents. Acrosin may represent as a uniquely druggable target due to its location and high cellular specificity. Thus, developing inhibitors of acrosin could provide the basis for safe, reversible male contraceptive
Birth control, also known as contraception, anticonception, and fertility control, is the use of methods or devices to prevent pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth control only be ...
s, or female contraceptives through the use of intravaginal contraceptive devices.
Moreover, as serine proteases are important in the potentiation of HIV
The human immunodeficiency viruses (HIV) are two species of '' Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the im ...
, research has found that an acrosin inhibitor, 4'-acetamidophenyl 4-guanidinobenzoate, possess the ability to inhibit HIV infection in virus-inoculated lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include T cells (for cell-mediated and cytotoxic adaptive immunity), B cells (for humoral, antibody-driven adaptive immunity), an ...
s. This suggests the further role of acrosin inhibitors as potentially viable agents in the prevention of HIV transmission.
References
Further reading
* * * * * * * * * * * * * * * * *External links
* TheMEROPS
MEROPS is an online database for peptidases (also known as proteases, proteinases and proteolytic enzymes) and their inhibitors. The classification scheme for peptidases was published by Rawlings & Barrett in 1993, and that for protein inhibito ...
online database for peptidases and their inhibitorsS01.223
* {{Portal bar, Biology, border=no EC 3.4.21