ACAM2000 on:
[Wikipedia]
[Google]
[Amazon]
 ACAM2000 is a
ACAM2000 is a
 ACAM2000 is a
ACAM2000 is a smallpox vaccine
The smallpox vaccine is used to prevent smallpox infection caused by the variola virus. It is the first vaccine to have been developed against a contagious disease. In 1796, British physician Edward Jenner demonstrated that an infection with th ...
and an mpox vaccine manufactured by Emergent Biosolutions
Emergent BioSolutions Inc. is an American multinational specialty biopharmaceutical company headquartered in Gaithersburg, Maryland. It develops vaccines and antibody therapeutics for infectious diseases and opioid overdoses, and it provides m ...
. It provides protection against smallpox
Smallpox was an infectious disease caused by Variola virus (often called Smallpox virus), which belongs to the genus '' Orthopoxvirus''. The last naturally occurring case was diagnosed in October 1977, and the World Health Organization (W ...
for people determined to be at high risk for smallpox infection. ACAM2000 is a live replicating vaccinia virus vaccine.
Medical uses
ACAM2000 isindicated
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis ...
for active immunization against smallpox disease for individuals determined to be at high risk for smallpox infection. It is also indicated for the active prevention of mpox
Mpox (, ; formerly known as monkeypox) is an infectious viral disease that can occur in humans and other animals. Symptoms include a rash that forms blisters and then crusts over, fever, and swollen lymph nodes. The illness is usually mild, ...
disease in individuals determined to be at high risk for mpox infection.
History
ACAM2000 is a vaccine developed by Acambis, which was acquired by Sanofi Pasteur in 2008, before selling the smallpox vaccine to Emergent Biosolutions in 2017. Six strains of ''vaccinia'' were isolated from 3,000 doses of Dryvax and found to exhibit significant variation in virulence. The strain with the most similar virulence to the overall Dryvax mixture was selected and grown in MRC-5 cells to make the ACAM1000 vaccine. After a successful Phase I trial of ACAM1000, the virus was passaged three times inVero cell
Vero cells are a lineage of cells used in cell cultures. The 'Vero' lineage was isolated from kidney epithelial cells extracted from an African green monkey ('' Chlorocebus'' sp.; formerly called ''Cercopithecus aethiops'', this group of monk ...
s to develop ACAM2000, which entered mass production at Baxter. The United States ordered over 200 million doses of ACAM2000 in 1999–2001 for its stockpile, and production is ongoing to replace expired vaccine.
Emergent Biosolutions developed ACAM2000 under a contract with the US Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) is the National public health institutes, national public health agency of the United States. It is a Federal agencies of the United States, United States federal agency under the United S ...
(CDC).
The US Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA) approved ACAM2000 in August 2007. By February 2008, it replaced Dryvax for all smallpox vaccinations.
As of 2010, there were over 200 million doses manufactured for the US Strategic National Stockpile.
According to the US FDA, "The approval and availability of this second-generation smallpox vaccine in the Strategic National Stockpile (SNS) enhances the emergency preparedness of the United States against the use of smallpox as a dangerous biological weapon
Biological agents, also known as biological weapons or bioweapons, are pathogens used as weapons. In addition to these living or replicating pathogens, toxins and Toxin#Biotoxins, biotoxins are also included among the bio-agents. More than 1,2 ...
."
In August 2024, ACAM2000 was approved for mpox prevention in the United States.
Administration of ACAM2000
The ACAM2000 vaccine is produced from thevaccinia
The vaccinia virus (VACV or VV) is a large, complex, enveloped virus belonging to the poxvirus family. It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of the ...
virus, which is sufficiently closely related to smallpox to provide immunity, but the ACAM2000 vaccine cannot cause smallpox because it does not contain the smallpox virus. Other vaccines containing live viruses include measles
Measles (probably from Middle Dutch or Middle High German ''masel(e)'', meaning "blemish, blood blister") is a highly contagious, Vaccine-preventable diseases, vaccine-preventable infectious disease caused by Measles morbillivirus, measles v ...
, mumps
MUMPS ("Massachusetts General Hospital Utility Multi-Programming System"), or M, is an imperative, high-level programming language with an integrated transaction processing key–value database. It was originally developed at Massachusetts Gen ...
, rubella
Rubella, also known as German measles or three-day measles, is an infection caused by the rubella virus. This disease is often mild, with half of people not realizing that they are infected. A rash may start around two weeks after exposure and ...
, polio
Poliomyelitis ( ), commonly shortened to polio, is an infectious disease caused by the poliovirus. Approximately 75% of cases are asymptomatic; mild symptoms which can occur include sore throat and fever; in a proportion of cases more severe ...
and chickenpox
Chickenpox, also known as varicella ( ), is a highly contagious disease caused by varicella zoster virus (VZV), a member of the herpesvirus family. The disease results in a characteristic skin rash that forms small, itchy blisters, which ...
.
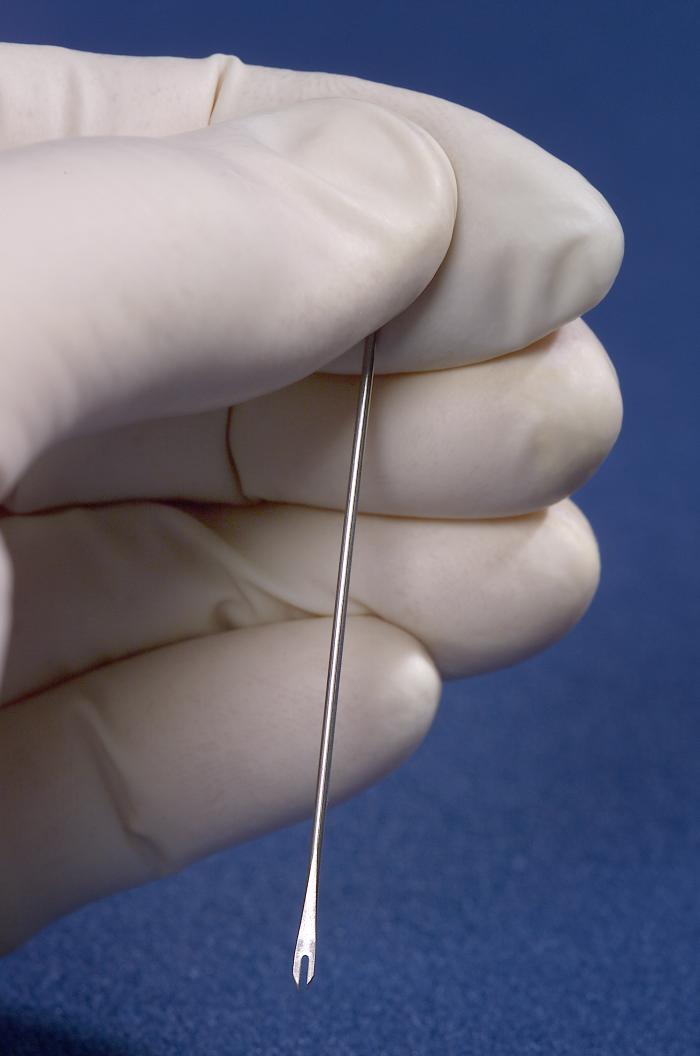
The vaccine is administered using a bifurcated stainless steel needle. The needle is dipped into the vaccine solution and used to prick the skin several times in the upper arm. The vaccinia virus will begin to grow at the injection site. It will cause a localized infection, with a red itchy sore produced at the vaccination site within three to four days. If the infection occurs, that is an indication that the vaccine was successful. Ultimately, the sore turns into a blister and then dries up. A scab forms and then falls off in the third week, leaving a small scar behind.
Risks
Administration of ACAM2000 poses risks and may cause side effects. Most people who have taken the vaccine only report mild reactions. Reactions may include a sore arm, fever, and body aches. Some people may have more serious side effects, including effects that may be life-threatening. According to the FDA-approved prescribing information leaflet, "Common adverse events include inoculation site signs and symptoms, lymphadenitis, and constitutional symptoms, such as malaise, fatigue, fever, myalgia, and headache." These reactions are less frequent in people being revaccinated than those receiving the vaccine for the first time. No known contraindications exist to receiving the vaccine in case of an outbreak emergency. Furthermore, it is recommended that the vaccine should be given to pregnant women who have been exposed to smallpox. "Because the risk of maternal serious illness or death, prematurity, miscarriage, or stillbirth from a smallpox infection are greater than the risk of the vaccination, smallpox vaccine is recommended and should be offered to pregnant women in case of an outbreak emergency."References
External links
* {{Authority control Healthcare in the United States History of immunology Infectious diseasesVaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifi ...
Smallpox vaccines
Vaccines