History of hydrogen isotopes
Earliest work
The study of Also in 1934, scientists
Also in 1934, scientists Impact on physical chemistry
The discovery of hydrogen isotopes also impacted In
In Impact on biogeochemistry
In biogeochemistry, scientists focused mainly on deuterium as a tracer for environmental processes, especially theImportant concepts
Stable vs radioactive isotopes
All isotopes of an element have the same number of protons with varying numbers of neutrons. Hydrogen has three naturally occurring isotopes: H, H and H; called protium (H), deuterium (D) and tritium (T), respectively. Both H and H are stable, while H isIsotope notation
The study of stable isotope biogeochemistry involves the description of the relative abundances of various isotopes in a certain chemical pool, as well as the way in which physicochemical processes change the fraction of those isotopes in one pool vs. another. Various type of notation have been developed to describe the abundance and change in the abundance of isotopes in these processes, and these are summarized below. In most cases only the relative amounts of an isotope are of interest, the absolute concentration of any one isotope is of little importance.Isotope ratio and fractional abundance
The most fundamental description of hydrogen isotopes in a system is the relative abundance of H and H. This value can be reported as isotope ratio R or fractional abundance F defined as: :Delta (öÇ) notation
Isotope ratios for a substance are often reported compared to a standard with known isotopic composition, and measurements of relative masses are always made in conjuncture with measuring a standard. For hydrogen, theMeasures of fractionation
The study of HIBGC relies on the fact that various physicochemical processes preferentially enrich or deplete H relative to H (seeConservation of mass in mixing calculations
H and H are stable isotopes. Therefore, the H/H ratio of a pool containing hydrogen, remains constant as long as no hydrogen is added or removed, a property known asNaturally occurring isotope variation
Natural processes result in broad variations in the D/H ratio (DHR) in different pools of hydrogen. KIEs and physical changes such as precipitation and evaporation lead to these observed variations. Seawater varies slightly, between 0 and ã10 per mil, while atmospheric water can vary between about ã200㯠to +100ã¯. Biomolecules synthesized by organisms, retain some of the D/H signature of the water which they were grown on, plus a large fractionation factor which can be as great as several hundred ã¯. Large D/H differences, of thousands of ã¯, can be found between Earth and other planetary bodies such as Mars, likely due to variations in isotope fractionation during planet formation and the loss of hydrogen into space.List of well known fractionation effects
A number of common processes fractionate hydrogen isotopes to produce the isotope variations found in nature. Common physical processes include precipitation and evaporation. Chemical reactions can also heavily influence the partitioning of heavy and light isotopes between pools. The rate of a chemical reaction depends in part on the energies of the chemical bonds formed and broken in the reaction. Since different isotopes have different masses, the bond energies differ betweenIsotope ratio as tracer for fingerprint
In many areas of study the origin of a chemical or group of chemicals is of central importance. Questions such as the source of environmental pollutants, the origin of hormones in an athlete's body, or the authenticity of foods and flavorings are all examples where chemical compounds need to be identified and sourced. Hydrogen isotopes have found uses in these and many other diverse areas of study. Since many processes can affect the DHR of a given compound this ratio can be a diagnostic signature for compounds produced in a specific location or via a certain process. Once the DHRs of a number of sources are known the measurement of this ratio for a sample of unknown origin can often be used to link it back to a certain source or production method.Physical chemistry
Hydrogen isotope formation
H, with oneQuantum properties
H is aKinetic and equilibrium isotope effects
Isotopes differ by number of Calculating the reduced mass of a HãH bond versus a HãH bond gives:
:
:
The quantum harmonic oscillator has energy levels of the following form, where ''k'' is the spring constant and ''h'' is the Planck constant.
:
The effects of this energy distribution manifest in the
Calculating the reduced mass of a HãH bond versus a HãH bond gives:
:
:
The quantum harmonic oscillator has energy levels of the following form, where ''k'' is the spring constant and ''h'' is the Planck constant.
:
The effects of this energy distribution manifest in the Chemistry of hydrogen exchange
One of the major complications in studying hydrogen isotopes is the issue of exchangeability. At many time scales, ranging from hours to geological epochs, scientists have to consider if the hydrogen moieties in studied molecules are the original species or if they represent exchange with water or mineral hydrogen near by. Research in this area is still inconclusive in regards to rates of exchange, but it is generally understood that hydrogen exchange complicates the preservation of information in isotope studies.Rapid exchange
Hydrogen atoms easily separate from Electronegativity, electronegative bonds such as hydroxyl bonds (OãH),Carbon bound hydrogen exchange
For some time, researchers believed that large hydrocarbon molecules were impervious to hydrogen exchange, but recent work has identified many reactions that allow isotope reordering. The isotopic exchange becomes relevant at geologic time scales and has impacted work of biologists studying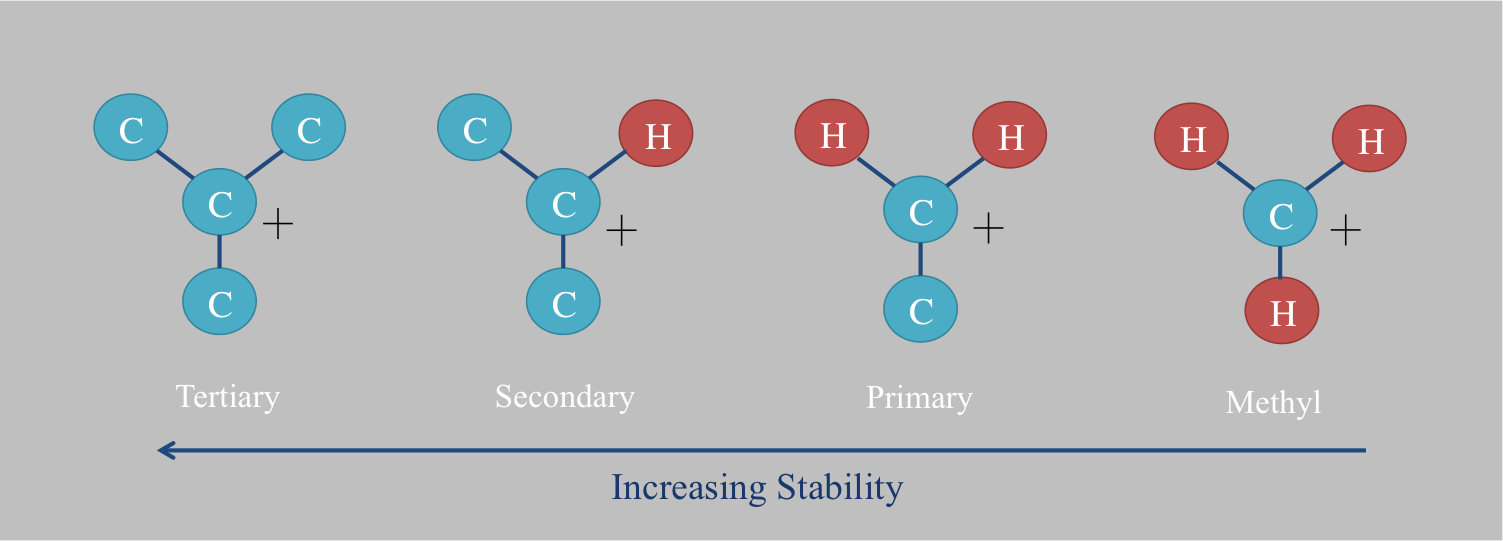 # Radical reactions that cleave CãH bonds.
# Ion exchange that of tertiary and aromatic hydrogen.
# Ketoãenol tautomerism, Enolizations that activate hydrogens on ketone Alpha and beta carbon, alpha carbons.
# Stereochemistry, Stereochemical exchange that causes Stereoinversion, stereochemical inversion.
# Constitutional exchange like Methyl group, methyl shifts, double bond migrations and carbon backbone rearrangements.
Detailed kinetics of these reactions have not been determined. However, it is known that clay minerals catalyze ionic hydrogen exchange faster than other minerals. Thus hydrocarbons formed in Clastic rock, clastic environments exchange more than those in Carbonate rock, carbonate settings. Aromatic and Tertiary hydrogen bond, tertiary hydrogen also have greater exchange rates than primary hydrogen. This is due to the increasing stability of associated carbocations. Primary carbocations are considered too unstable to exist and have never been isolated in an Fourier transform ion cyclotron resonance, FT-ICR spectrometer. On the other hand, tertiary carbocations are relatively stable and are often Reaction intermediate, intermediates in organic chemistry reactions. This stability, which increases the likelihood of proton loss, is due to the Electron donating group, electron donation of nearby carbon atoms. Resonance (chemistry), Resonance and nearby lone pairs can also stabilize carbocations via Electron donating group, electron donation. Aromatic hydrocarbon, Aromatic carbons are thus relatively easy to exchange.
Many of these reactions have a strong temperature dependence; higher temperature typically accelerates exchange. However, different mechanisms may prevail at each temperature window. Ion exchange, for example, is most significant at low temperature. In such low-temperature environments, there is potential for preserving the original hydrogen isotope signal over hundreds of millions of years. However, many rocks in Geologic time scale, geologic time have reached significant Maturity (geology), thermal maturity. Even by the onset of the oil window it appears that much of the hydrogen has exchanged. Recently, scientists have explored a silver lining: hydrogen exchange is a Zero order reaction, zero order kinetic reaction (for carbon bound hydrogen at 80ã100ô¯C, the Half time (physics), half-times are likely 10ã10 years). Applying the mathematics of Reaction rate constant, rate constants would allow extrapolation to original isotopic compositions. While this solution holds promise, there is too much disagreement in the literature for robust calibrations.
# Radical reactions that cleave CãH bonds.
# Ion exchange that of tertiary and aromatic hydrogen.
# Ketoãenol tautomerism, Enolizations that activate hydrogens on ketone Alpha and beta carbon, alpha carbons.
# Stereochemistry, Stereochemical exchange that causes Stereoinversion, stereochemical inversion.
# Constitutional exchange like Methyl group, methyl shifts, double bond migrations and carbon backbone rearrangements.
Detailed kinetics of these reactions have not been determined. However, it is known that clay minerals catalyze ionic hydrogen exchange faster than other minerals. Thus hydrocarbons formed in Clastic rock, clastic environments exchange more than those in Carbonate rock, carbonate settings. Aromatic and Tertiary hydrogen bond, tertiary hydrogen also have greater exchange rates than primary hydrogen. This is due to the increasing stability of associated carbocations. Primary carbocations are considered too unstable to exist and have never been isolated in an Fourier transform ion cyclotron resonance, FT-ICR spectrometer. On the other hand, tertiary carbocations are relatively stable and are often Reaction intermediate, intermediates in organic chemistry reactions. This stability, which increases the likelihood of proton loss, is due to the Electron donating group, electron donation of nearby carbon atoms. Resonance (chemistry), Resonance and nearby lone pairs can also stabilize carbocations via Electron donating group, electron donation. Aromatic hydrocarbon, Aromatic carbons are thus relatively easy to exchange.
Many of these reactions have a strong temperature dependence; higher temperature typically accelerates exchange. However, different mechanisms may prevail at each temperature window. Ion exchange, for example, is most significant at low temperature. In such low-temperature environments, there is potential for preserving the original hydrogen isotope signal over hundreds of millions of years. However, many rocks in Geologic time scale, geologic time have reached significant Maturity (geology), thermal maturity. Even by the onset of the oil window it appears that much of the hydrogen has exchanged. Recently, scientists have explored a silver lining: hydrogen exchange is a Zero order reaction, zero order kinetic reaction (for carbon bound hydrogen at 80ã100ô¯C, the Half time (physics), half-times are likely 10ã10 years). Applying the mathematics of Reaction rate constant, rate constants would allow extrapolation to original isotopic compositions. While this solution holds promise, there is too much disagreement in the literature for robust calibrations.
Vapor isotope effects
Vapor isotope effects occur for H, H, and H; since each isotope has different thermodynamic properties in the liquid and gas phases. For water, the condensed phase is more enriched while the vapor is more depleted. For example, rain condensing from a cloud, is heavier than the vapor starting point. Generally, the large variations in deuterium concentration in water are from fractionations between liquid, vapor, and solid reservoirs. In contrast to the fractionation pattern of water, non-polar molecules like oils and lipids, have gaseous counterparts enriched with deuterium relative to the liquid. This is thought to be associated with the polarity from hydrogen bonding in water that does not interfere in long-chain hydrocarbons.Observed variations in isotope abundance
 Due to physical and chemical fractionation processes, the variations in the isotopic compositions of elements are reported, and the standard atomic weights of hydrogen isotopes have been published by IUPAC's Commission on Atomic Weights and Isotopic Abundances. The HICs are reported relative to the International Atomic Energy Agency (IAEA) reference water. In the equilibrium isotope reactions of H/H in general, enrichment of the heavy isotope is observed in the compound with the higher oxidation state. However, in our natural environment, HIC varies greatly depending on the sources and organisms due to complexities of interacting elements in disequilibrium states. In this section, the observed variations in HIC of water sources (hydrosphere), living organisms (biosphere), organic substances (geosphere), and extraterrestrial materials in the Solar system are described.
Due to physical and chemical fractionation processes, the variations in the isotopic compositions of elements are reported, and the standard atomic weights of hydrogen isotopes have been published by IUPAC's Commission on Atomic Weights and Isotopic Abundances. The HICs are reported relative to the International Atomic Energy Agency (IAEA) reference water. In the equilibrium isotope reactions of H/H in general, enrichment of the heavy isotope is observed in the compound with the higher oxidation state. However, in our natural environment, HIC varies greatly depending on the sources and organisms due to complexities of interacting elements in disequilibrium states. In this section, the observed variations in HIC of water sources (hydrosphere), living organisms (biosphere), organic substances (geosphere), and extraterrestrial materials in the Solar system are described.
Hydrosphere
Oceans
 Variations in öÇD of different water sources and ice caps are observed due to evaporation and condensation processes. (See section 6 for more details.) When seawater is well-mixed, the öÇD at equilibrium is near 0㯠(㯠SMOW) with a DHR of 155.76 ppm. However, continuous variations in öÇD are caused by evaporation or precipitation processes which lead to disequilibrium in fractionation processes. A large HIC gradient occurs in surface waters of the oceans, and the fluctuation value in the Northwest Atlantic surface water is around 20ã¯. According to the data examining the southern supersegment of the Pacific Ocean, as latitude decreases from 65ùS to 40ùS, öÇD fluctuates between around ã50㯠and ã70ã¯.
The HIC of seawater (not just surface water) is mostly in the range of 0㯠to ã10ã¯. The estimates of öÇD for different parts of the ocean across the world are shown on the map.
Variations in öÇD of different water sources and ice caps are observed due to evaporation and condensation processes. (See section 6 for more details.) When seawater is well-mixed, the öÇD at equilibrium is near 0㯠(㯠SMOW) with a DHR of 155.76 ppm. However, continuous variations in öÇD are caused by evaporation or precipitation processes which lead to disequilibrium in fractionation processes. A large HIC gradient occurs in surface waters of the oceans, and the fluctuation value in the Northwest Atlantic surface water is around 20ã¯. According to the data examining the southern supersegment of the Pacific Ocean, as latitude decreases from 65ùS to 40ùS, öÇD fluctuates between around ã50㯠and ã70ã¯.
The HIC of seawater (not just surface water) is mostly in the range of 0㯠to ã10ã¯. The estimates of öÇD for different parts of the ocean across the world are shown on the map.
Ice caps
 Typical öÇDs for ice sheets in the polar regions range from around ã400㯠to ã300㯠(ã¯SMOW). Ice caps' öÇDs are affected by distance from open ocean, latitude, atmospheric circulation, and the amount of insolation and temperature. The temperature change affects the HIC of ice caps, so the HIC of ice can give estimates for the historical climate cycles such as the timelines for interglacial and glacial periods. [See section 7.2. Paleo-reconstruction for more details]
The öÇDs of ice caps from 70 km south of Vostok Station and in East Antarctica are ã453.7㯠and ã448.4㯠respectively, and are shown on the map.
Typical öÇDs for ice sheets in the polar regions range from around ã400㯠to ã300㯠(ã¯SMOW). Ice caps' öÇDs are affected by distance from open ocean, latitude, atmospheric circulation, and the amount of insolation and temperature. The temperature change affects the HIC of ice caps, so the HIC of ice can give estimates for the historical climate cycles such as the timelines for interglacial and glacial periods. [See section 7.2. Paleo-reconstruction for more details]
The öÇDs of ice caps from 70 km south of Vostok Station and in East Antarctica are ã453.7㯠and ã448.4㯠respectively, and are shown on the map.
Atmosphere
 The analysis done based on satellite measurement data, estimates öÇD for the air in various parts of the world. The general trend is that öÇD is more negative at higher latitude, so air above Antarctica and the Arctic is D-depleted to around ã230㯠to ã260㯠or even lower.
The estimated atmospheric öÇDs are shown on the map.
A vast portion of global atmospheric water vapor comes from the Western Pacific near the tropics, (mean 2009) and the HIC of air depends on temperature and humidity. Hot, humid regions generally have higher öÇD. Water vapor in the air is in general more depleted than terrestrial water sources, since HO evaporates faster than HHO due to higher vapor pressure. On the other hand, rain water is in general more enriched than atmospheric water vapor.
The analysis done based on satellite measurement data, estimates öÇD for the air in various parts of the world. The general trend is that öÇD is more negative at higher latitude, so air above Antarctica and the Arctic is D-depleted to around ã230㯠to ã260㯠or even lower.
The estimated atmospheric öÇDs are shown on the map.
A vast portion of global atmospheric water vapor comes from the Western Pacific near the tropics, (mean 2009) and the HIC of air depends on temperature and humidity. Hot, humid regions generally have higher öÇD. Water vapor in the air is in general more depleted than terrestrial water sources, since HO evaporates faster than HHO due to higher vapor pressure. On the other hand, rain water is in general more enriched than atmospheric water vapor.
Precipitation
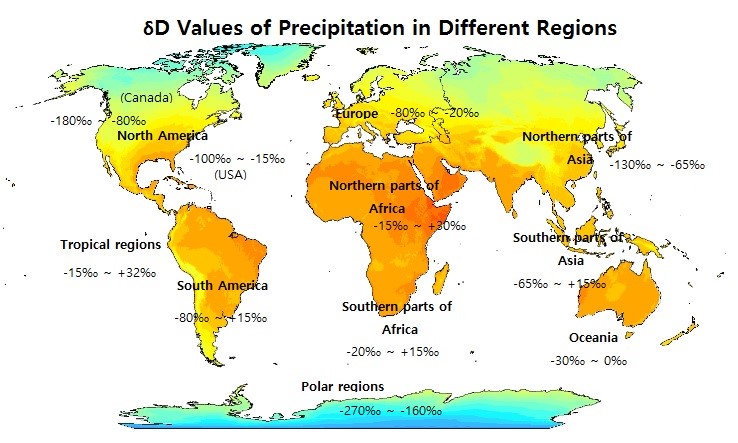 öÇDs of annual precipitation in different regions of the world are shown on the map. The precipitation is more D-enriched near the equator in the Tropics. The öÇDs generally fall in the range of around ã30 ~ ã150㯠in the northern hemisphere and ã30~+30㯠over land areas of the southern hemisphere. In North America, the öÇD of average monthly precipitation across regions is lower in January (ranging up to around ã300㯠in Canada) than in July (up to around ã190ã¯).
The overall mean precipitation is determined by the balance between evaporation of water from the oceans and other surface water and condensation of water vapor in the form of rain. Net evaporation should equal net precipitation, and the öÇD for precipitation is around ã22㯠(global average). The Global Network of Isotopes in Precipitation (GNIP) investigates and monitors the isotopic composition of precipitation at various sites all over the world. The mean precipitation can be estimated by the equation, öÇH = 8.17(ôÝ0.07) öÇO + 11.27(ôÝ0.65)㯠VSMOW. (Rozanski et al., 1993) This equation is the slightly modified version from the general global meteoric water line (GMWL) equation, öÇH = 8.13öÇO + 10.8, which provides the average relationship between öÇH and öÇO of natural terrestrial waters.
öÇDs of annual precipitation in different regions of the world are shown on the map. The precipitation is more D-enriched near the equator in the Tropics. The öÇDs generally fall in the range of around ã30 ~ ã150㯠in the northern hemisphere and ã30~+30㯠over land areas of the southern hemisphere. In North America, the öÇD of average monthly precipitation across regions is lower in January (ranging up to around ã300㯠in Canada) than in July (up to around ã190ã¯).
The overall mean precipitation is determined by the balance between evaporation of water from the oceans and other surface water and condensation of water vapor in the form of rain. Net evaporation should equal net precipitation, and the öÇD for precipitation is around ã22㯠(global average). The Global Network of Isotopes in Precipitation (GNIP) investigates and monitors the isotopic composition of precipitation at various sites all over the world. The mean precipitation can be estimated by the equation, öÇH = 8.17(ôÝ0.07) öÇO + 11.27(ôÝ0.65)㯠VSMOW. (Rozanski et al., 1993) This equation is the slightly modified version from the general global meteoric water line (GMWL) equation, öÇH = 8.13öÇO + 10.8, which provides the average relationship between öÇH and öÇO of natural terrestrial waters.
Lakes and rivers
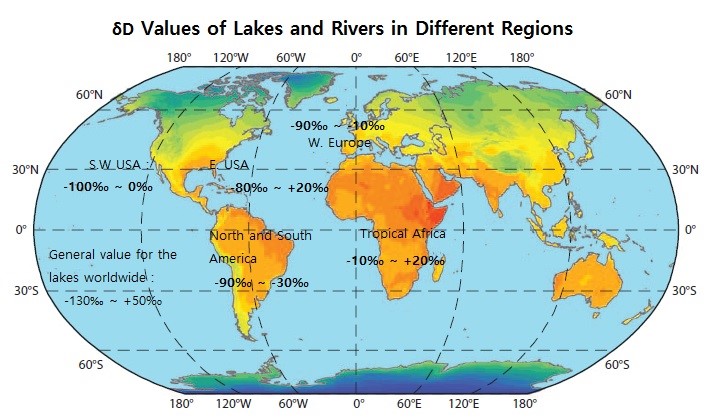 The öÇDs vs. VSMOW of lakes in different regions are shown on the map. The general pattern observed, indicates that öÇDs of surface waters including lakes and rivers, are similar to that of local precipitation.See Section 7.1. Isotope hydrology for more details.
The öÇDs vs. VSMOW of lakes in different regions are shown on the map. The general pattern observed, indicates that öÇDs of surface waters including lakes and rivers, are similar to that of local precipitation.See Section 7.1. Isotope hydrology for more details.
Soil water
The isotopic composition of soil is controlled by the input of precipitation. Therefore, the öÇD of soil is similar to that of local precipitation. However, due to evaporation, soil tends to be more D-enriched than precipitation. The degree of enrichment varies greatly depending on atmospheric humidity, local temperature as well as the depth of the soil beneath the surface. According to the study by Meinzer et al. (1999), as the depth in the soil increases, the öÇD of soil water decreases.Biosphere
Marine algae
The factors affecting öÇD of algalPhytoplankton and bacteria
The öÇD of lipids from phytoplankton is largely affected by öÇD of water, and there seems to be a linear correlation between those two values. The öÇD of most other biosynthetic products in phytoplankton or cyanobacteria are more negative than that of the surrounding water. The öÇD values of fatty acids in methanotrophs living in seawater lie between ã50 and ã170ã¯, and that of sterols and hopanols range between ã150 and ã270ã¯.Sessions, A.L. (2002) Hydrogen isotope fractionation in lipids of the methane-oxidizing bacterium Methylococcus capsulatus. Geochimica et Cosmochimica Acta, 66. 22: 3955ã3969. The HIC of photoautotrophs can be estimated using the equation, : , where , and are the DHRs of lipids, water, and substrates, respectively. is the mole fraction of lipid H derived from external water, whereas and denote the net isotopic fractionations associated with uptake and utilization of water and substrate hydrogen, respectively. For phototrophs, is calculated assuming that = 1. The isotopic fractionation between lipids and= öÇD values for lipids in bacterial species
= Source: * Lipids in organisms growing on heterotrophic substrates: ** Growing on sugar: depleted 200㯠~ 300㯠relative to water ** Growing on direct precursor of TCA cycle (e.g. acetate (öÇD = ã76ã¯) or succinate): enriched ã50㯠~ +200㯠relative to water ** : ã150㯠~ +200㯠* Lipids in organisms growing photoautotrophically: ** Depleted 50㯠~ 190㯠relative to water ** : ã150㯠~ ã250㯠* Lipids in organisms growing chemoautotrophically: ** : ã200㯠~ ã400ã¯Plants
 öÇDs for n-C alkane(ã¯) vs. VSMOW for different plant groups are as follows. Here, represents öÇDs for n-C alkane(ã¯) vs. VSMOW, and represents öÇDs for mean annual precipitation (ã¯) vs. VSMOW).
For plant leaf wax, the relative humidity, the timing of leaf wax formation and the growth conditions including light levels affect the D/H fractionation of plant wax. From the CraigãGordon model, it can be understood that leaf water in the growth chamber gasses is significantly D-enriched due to transpiration.
öÇDs for n-C alkane(ã¯) vs. VSMOW for different plant groups are as follows. Here, represents öÇDs for n-C alkane(ã¯) vs. VSMOW, and represents öÇDs for mean annual precipitation (ã¯) vs. VSMOW).
For plant leaf wax, the relative humidity, the timing of leaf wax formation and the growth conditions including light levels affect the D/H fractionation of plant wax. From the CraigãGordon model, it can be understood that leaf water in the growth chamber gasses is significantly D-enriched due to transpiration.
= Sugars
= The global abundance of H in plants is in the following order: phenylpropanoids > carbohydrates > bulk material > hydrolyzable lipids > steroids. In plants, öÇDs of carbohydrates, which typically range around ã70㯠to ã140ã¯, are good indicators of the photosynthetic metabolism. Photosynthetically produced hydrogen which is bound to carbon backbones is ~100ã¯ã170㯠more D-depleted than the water in plant tissues. Heterotrophic processing of carbohydrates involves isomerization of triose phosphates and interconversion between fructose-6-phosphate and glucose-6-phosphate. These cellular processes promote the exchange between organic H and within the plant tissues leading to around 158㯠of D-enrichment of those exchanged sites.Sessions, A.L. et al. (1999) Fractionation of hydrogen isotopes in lipid biosynthesis. Organic Geochemistry. 30. 1193ã1200 The öÇD of C3 plant, plants such as sugar beet, orange and grape ranges from ã132㯠to ã117ã¯, and that of C4 plant, plants such as sugar cane and maize ranges from ã91㯠to ã75ã¯. The öÇD of Crassulacean acid metabolism (CAM) such as pineapple is estimated at around ã75ã¯. Sugar beet and sugar cane contain sucrose, and maize contain glucose. Orange and pineapple are the sources of glucose and fructose. The deuterium content of the sugars from the above plant species are not distinctive. In plants, hydrogen attached to carbons in 4 and 5 positions of the glucose typically comes from NADPH in the photosynthetic pathway, and is found to be more D-enriched. Whereas in plants, hydrogen attached to carbons 1 and 6 positions is more D-enriched. D-enrichment patterns in CAM species tend to be closer to that in species.= Bulk organic matter
= The HIC of leaf water is variable during the biosynthesis, and the enrichment in the whole leaf can be described by the equation, ã°D = ã°D û ([1 ã e]/P) The typical öÇD of bulk plant is around ã160ã¯, while öÇDs forAnimals
HIC in animal tissues is hard to estimate due to complexities in the diet intake and the isotopic composition of surrounding water sources. When fish species were investigated, average HIC of proteins was in a large range of ã128㯠~ +203ã¯. In the bulk tissue of organisms, all lipids were found to be D-depleted, and the values of öÇD for lipids tend to be lower than that for proteins. The average öÇD for Chironomid and fish protein was estimated to be in the range of ã128㯠to +203ã¯.Soto, D. X., Wassenaar, L. I., Hobson, K. A., (2013) Stable hydrogen and oxygen isotopes in aquatic food webs are tracers of diet and provenance. Functional Ecology. Vol. 27. Issue. 2. 535ã543. Most hydrogen in heterotrophic tissues comes from water not from diet sources, but the proportion coming from water varies. In general, hydrogen from water is transferred to NADPH and then taken up to the tissues. An apparent trophic species, trophic effect (compounding effect) can be observed for öÇD in heterotrophs, so significant D-enrichments result from the intake of surrounding water the in aquatic food webs. The öÇD of proteins in animal tissues are in cases affected more by diet sources than by surrounding water. Though different öÇDs for the same class of compounds may arise in different organisms growing in water with the same öÇD, those compounds generally have the same öÇD within each organism itself. [See Section 7.5. Ecology for more details]= Lipids
= öÇDs of fatty acids in living organisms, are typically ã73㯠to ã237ã¯. The öÇDs of individual fatty acids vary widely between cultures (ã362㯠to +331ã¯), but typically by less than around 30㯠between different fatty acids from the same species. The differences in öÇD for the compounds within the same lipid class is generally less than 50ã¯, whereas the difference falls in the range of 50ã¯ã150㯠for the compounds in different lipid classes. öÇDs for typical lipid groups are determined using the following equation: : ; where = net or apparent fractionation, = lipid product and = source water. * The öÇDs of common lipid classes found in living organisms are: ** n-alkyl: ã170㯠ôÝ 50㯠(113ã¯ã262㯠more D-depleted than growth water) ** isoprenoid: ã270㯠ôÝ 75㯠(142ã¯ã376㯠more D-depleted than growth water) ** phytol: ã360㯠ôÝ 50㯠(more depleted than the other two categories) Polyisoprenoid lipids are more depleted than acetogenic (n-alkyl) lipids with more negative öÇDs. ãÀ Enrichment relative to waterGeosphere
Oil
Source: * Oil samples from northeast Japan: from ã130㯠to around ã110㯠with higher maturity. * Oil samples from Portiguar Basin: ã90㯠(lancustrine environment), ã120㯠to ã135㯠(marine-evaporitic environment),Alkenones
 The isotopic composition of alkenones often reflect the isotopic enrichment or depletion of the surrounding environment, and öÇDs of alkenones in different regions are shown on the map.
The isotopic composition of alkenones often reflect the isotopic enrichment or depletion of the surrounding environment, and öÇDs of alkenones in different regions are shown on the map.
Coals
 Source:
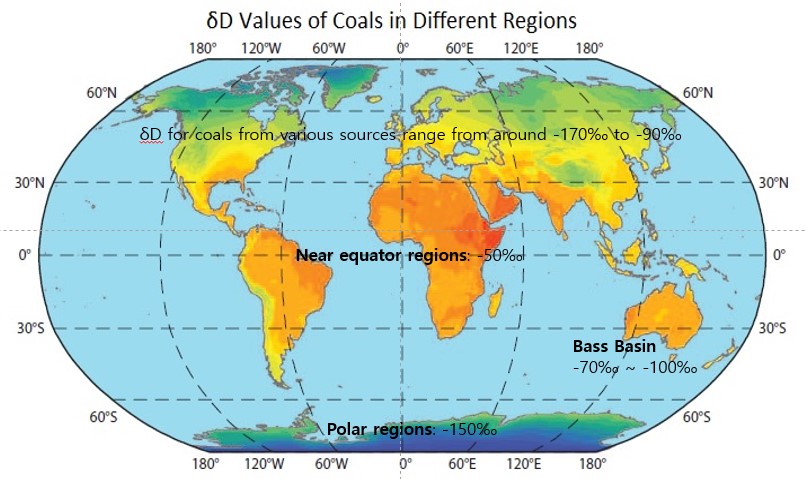
According to the studies by Reddings et al., öÇDs for coals from various sources range from around ã90㯠to ã170ã¯.
The öÇDs of coals in different regions are shown on the map.
Source:
According to the studies by Reddings et al., öÇDs for coals from various sources range from around ã90㯠to ã170ã¯.
The öÇDs of coals in different regions are shown on the map.
Natural gas
Source:= Methane
= Methane produced by marine methanogens is typically more D-enriched than methane produced by methanogens grown in freshwater. öÇDs for thermogenic methane range from ã275㯠to ã100ã¯, and from ã400㯠to ã150㯠for microbial methane.H2 gas
The öÇD of atmospheric H is around +180ã¯, the biggest öÇD known for natural terrestrials (mole fraction H: 183.8 ppm). The öÇD ofMineral H
 The öÇDs of hydroxyl-bearing minerals of the mantle were estimated at ã80㯠~ ã40㯠via analysis of the isotopic composition for juvenile water. Hydrogen minerals generally have large isotope effects, and the isotopic composition often follows the pattern observed for precipitation.
The öÇDs of hydroxyl-bearing minerals of the mantle were estimated at ã80㯠~ ã40㯠via analysis of the isotopic composition for juvenile water. Hydrogen minerals generally have large isotope effects, and the isotopic composition often follows the pattern observed for precipitation.
= Clay minerals
= The D/H fractionations in clays such as kaolinite, illite, smectite are in most cases consistent when no significant external forces are applied under constant temperature and pressure. The following is an empirically determined equation for estimating the D/H fractionation factor: 1000 In öÝ = ã2.2 û 10 û T ã 7.7. The öÇDs vs. ã¯SMOW for hydrogen minerals found in mantle (geology), mantle, metamorphic rock, shales, marine clays, marine carbonates and sedimentary rocks are shown in the table.Extraterrestrial objects
Variations of DHR in the Solar System ;Earth
:The HIC of mantle (geology), mantle rocks on Earth is highly variable; and that of mantle water is around ã80㯠~ ã50㯠depending on its states such as fluid, hydrous phase, hydroxyl point defect, juvenile water (from degassing of the mantle), magmatic water (water equilibrated with a magma).
;Sun
:The Sun's DHR is around 21 ôÝ 5 û 10.
;Mars
:The current HIC is enriched by a factor of 5 relative to Earth's seawater due to continual losses of H in Martian atmosphere. Therefore, the öÇD is estimated at around +4000ã¯.
The DHRs of Jupiter and Saturn are nearly in the order of 10, and the DHRs of Uranus and Neptune are closer to 10.
Hydrogen is the most abundant element in the universe. Variations in isotopic composition of extraterrestrial materials stem from planetary accretion (astrophysics), accretion or other planetary processes such as atmospheric escape, and are larger for H and N than for C and O. The preservation of D-enrichment is observed in chondritic meteorites, interplanetary medium, interplanetary dust particles and cometary Volatile (astrogeology), Volatiles.
From the
;Earth
:The HIC of mantle (geology), mantle rocks on Earth is highly variable; and that of mantle water is around ã80㯠~ ã50㯠depending on its states such as fluid, hydrous phase, hydroxyl point defect, juvenile water (from degassing of the mantle), magmatic water (water equilibrated with a magma).
;Sun
:The Sun's DHR is around 21 ôÝ 5 û 10.
;Mars
:The current HIC is enriched by a factor of 5 relative to Earth's seawater due to continual losses of H in Martian atmosphere. Therefore, the öÇD is estimated at around +4000ã¯.
The DHRs of Jupiter and Saturn are nearly in the order of 10, and the DHRs of Uranus and Neptune are closer to 10.
Hydrogen is the most abundant element in the universe. Variations in isotopic composition of extraterrestrial materials stem from planetary accretion (astrophysics), accretion or other planetary processes such as atmospheric escape, and are larger for H and N than for C and O. The preservation of D-enrichment is observed in chondritic meteorites, interplanetary medium, interplanetary dust particles and cometary Volatile (astrogeology), Volatiles.
From the Measurement techniques
DHR can be determined with a combination of different preparation techniques and instruments for different purposes. There are several types of HIC measurement: (i) organic hydrogen or water are converted to H first, followed by high-precision isotope-ratio mass spectrometry (IRMS) measurement; (ii) H/H and O/O are directly measured as HO by laser spectroscopy also with high precision; (iii) the intact molecules are directly measured by NMR orOffline combustion and reduction
Conversion to simple molecules (i.e. H for hydrogen) is required prior to IRMS for stable isotopes. This is for several reasons with regard to hydrogen: The classical offline preparation for the conversion is combustion over CuO at >800ô¯C in sealed quartz tubes, followed by the isolation of resulting water and the reduction to H over hot metal at 400 ~1000ô¯C on a vacuum line. The produced gas is then directly injected into the dual-inlet mass spectrometer for measurement. The metals used for reduction to H includes U, Zn, Cr, Mg and Mn, etc. U and Zn had been widely used since the 1950s until Cr was successfully employed in the late 1990s. The offline combustion/reduction has the highest accuracy and precision for HIC measurement without limits for sample types. The analytical uncertainty is typically 1~2㯠in öÇD. Thus it is still used today when highest levels of precision are required. However, the offline preparation procedure is very time-consuming and complicated. It also requires a large sample (several 100 mg). Thus, online preparation based on combustion/reduction coupled with the subsequent continuous flow-IRMS (CF-IRMS) system has been more often used nowadays. Chromium reduction or high temperature conversion are the dominant online preparation methods for detection of HIC by IRMS.High temperature conversion/elemental analyzer (TC/EA)
 TC/EA (or HTC, high temperature conversion; HTP, high temperature pyrolysis; HTCR, high temperature carbon reduction) is an "online" or "continuous flow" preparation method typically followed by IRMS detection. This is a "bulk" technique that measures all the hydrogen in a sample and provides the average isotope signal. The weighed sample is placed in a tin or silver capsule and dropped into a pyrolysis tube of TC/EA. The tube is made of glassy carbon with glassy carbon filling, so oxygen isotopes can be measured simultaneously without oxygen exchange with ceramic (AlO) surface. The molecules are then reduced into CO and H at high temperature (>1400ô¯C) in the reactor. The gaseous products are separated through gas chromatography (GC) using helium as the carrier gas, followed by a split-flow interface, and finally detected by IRMS. TC/EA method can be problematic for organic compounds with halogen or nitrogen due to the competition between the pyrolysis byproducts (e.g. HCl and HCN) and H formation. In addition, it is susceptible to contamination with water, so samples must be scrupulously dried.
An adaption of this method is to determine the non-exchangeable (C-H) and exchangeable hydrogen (bounds to other elements, e.g. O, S and N) in organic matter. The samples are equilibrated with water in sealed autosampler carousels at 115ô¯C and then transferred into pyrolysis EA followed by IRMS measurement.
TC/EA method is quick with fairly high precision (~1ã¯). It was limited to solid samples; however, liquid sample recently can also be measured in TC/EA-IRMS system by adapting an autosampler for liquids. The drawback of TC/EA is the relatively big sample size (~ mg), which is smaller than offline combustion/reduction but larger than GC/pyrolysis. It cannot separate different compounds as GC/pyrolysis does and thus only the average for the whole sample can be provided, which is also a drawback for some research.
TC/EA (or HTC, high temperature conversion; HTP, high temperature pyrolysis; HTCR, high temperature carbon reduction) is an "online" or "continuous flow" preparation method typically followed by IRMS detection. This is a "bulk" technique that measures all the hydrogen in a sample and provides the average isotope signal. The weighed sample is placed in a tin or silver capsule and dropped into a pyrolysis tube of TC/EA. The tube is made of glassy carbon with glassy carbon filling, so oxygen isotopes can be measured simultaneously without oxygen exchange with ceramic (AlO) surface. The molecules are then reduced into CO and H at high temperature (>1400ô¯C) in the reactor. The gaseous products are separated through gas chromatography (GC) using helium as the carrier gas, followed by a split-flow interface, and finally detected by IRMS. TC/EA method can be problematic for organic compounds with halogen or nitrogen due to the competition between the pyrolysis byproducts (e.g. HCl and HCN) and H formation. In addition, it is susceptible to contamination with water, so samples must be scrupulously dried.
An adaption of this method is to determine the non-exchangeable (C-H) and exchangeable hydrogen (bounds to other elements, e.g. O, S and N) in organic matter. The samples are equilibrated with water in sealed autosampler carousels at 115ô¯C and then transferred into pyrolysis EA followed by IRMS measurement.
TC/EA method is quick with fairly high precision (~1ã¯). It was limited to solid samples; however, liquid sample recently can also be measured in TC/EA-IRMS system by adapting an autosampler for liquids. The drawback of TC/EA is the relatively big sample size (~ mg), which is smaller than offline combustion/reduction but larger than GC/pyrolysis. It cannot separate different compounds as GC/pyrolysis does and thus only the average for the whole sample can be provided, which is also a drawback for some research.
Gas chromatography/pyrolysis (GC/pyrolysis)
 GC-interface (combustion or pyrolysis) is also an online preparation method followed by IRMS detection. This is a 'compound-specific' method, allowing separation of analytes prior to measurement and thus providing information about the isotopic composition of each individual compound. After GC separation, samples are converted to smaller gaseous molecules for isotope measurements. GC/pyrolysis uses the pyrolysis interface between GC and IRMS for the conversion of H and O in the molecules into H and CO. GC-IRMS was first introduced by Matthews and Hayes in the late 1970s, and was later used for öÇC, öÇN, öÇO and öÇS. Helium is used as the carrier gas in the GC systems. However, the separation of DH (m/z=3) signal from the tail of He beam was problematic due to the intense signal of He. During the early 1990s, intense efforts were made in solving the difficulties to measure öÇD by GC/pyrolysis-IRMS. In 1999, Hilkert et al. developed a robust method by integrating the high temperature conversion (TC) into GC-IRMS and adding a pre-cup electrostatic sector and a retardation lens in front of the m/z=3 cup collector. Several different groups were working on this at the same time. This GC/pyrolysis-IRMS based on TC has been widely used for öÇD measurement nowadays. The commercial products of GC-IRMS include both combustion and pyrolysis interfaces so that öÇC and öÇD can be measured simultaneously.
The significant advantage of GC/pyrolysis method for HIC measurement is that it can separate different compounds in the samples. It requires the smallest sample size (typically ~200 ng) relative to other methods and has a high precision of 1~5 ã¯. But this method is relatively slow and limited to the samples which can be applied in GC system.
GC-interface (combustion or pyrolysis) is also an online preparation method followed by IRMS detection. This is a 'compound-specific' method, allowing separation of analytes prior to measurement and thus providing information about the isotopic composition of each individual compound. After GC separation, samples are converted to smaller gaseous molecules for isotope measurements. GC/pyrolysis uses the pyrolysis interface between GC and IRMS for the conversion of H and O in the molecules into H and CO. GC-IRMS was first introduced by Matthews and Hayes in the late 1970s, and was later used for öÇC, öÇN, öÇO and öÇS. Helium is used as the carrier gas in the GC systems. However, the separation of DH (m/z=3) signal from the tail of He beam was problematic due to the intense signal of He. During the early 1990s, intense efforts were made in solving the difficulties to measure öÇD by GC/pyrolysis-IRMS. In 1999, Hilkert et al. developed a robust method by integrating the high temperature conversion (TC) into GC-IRMS and adding a pre-cup electrostatic sector and a retardation lens in front of the m/z=3 cup collector. Several different groups were working on this at the same time. This GC/pyrolysis-IRMS based on TC has been widely used for öÇD measurement nowadays. The commercial products of GC-IRMS include both combustion and pyrolysis interfaces so that öÇC and öÇD can be measured simultaneously.
The significant advantage of GC/pyrolysis method for HIC measurement is that it can separate different compounds in the samples. It requires the smallest sample size (typically ~200 ng) relative to other methods and has a high precision of 1~5 ã¯. But this method is relatively slow and limited to the samples which can be applied in GC system.
Laser spectroscopy
 Laser spectroscopy (or cavity ring-down spectroscopy, CRDS) is able to directly measure H/H, O/O and O/O isotopic composition in water or methane. The use of laser spectroscopy on hydrogen isotopes was first reported by Bergamaschi et al. in 1994. They directly measured CHD/CH in atmospheric methane using a lead salt tunable diode laser spectroscopy. The development of CRDS was first reported by O'Keefe et al. in 1988. In 1999, Kerstel et al. successfully applied this technique to determine HIC in water. The system consists of a laser and a Optical cavity, cavity equipped with high finesse reflectivity mirrors. Laser light is injected into the cavity, where the resonance takes place due to the constructive interference. The laser then is turned off. The decay of light intensity is measured. In the presence of a water sample, the photo-absorption by water isotopologues follows the kinetic law. The optical spectrum is obtained by recording ring-down time of the HO spectral features of interest at certain laser wavelength. The concentration of each isotopologue is proportional to the area under each measured isotopologue spectral feature.
Laser spectroscopy is quick, simple, and relatively cheap; and the equipment is portable. So it can be used in the field for measuring water samples. H/H and O/O can be determined simultaneously from a single injection. It requires a small sample size, < 1 ö¥L for water. Typical precision is ~ 1ã¯. However, this is a compound-specific instrument, i.e. only one specific compound can be measured. And coexisting organic compounds (i.e. ethanol) could interfere with the optical light absorption features of water, resulting in cross-contamination.
Laser spectroscopy (or cavity ring-down spectroscopy, CRDS) is able to directly measure H/H, O/O and O/O isotopic composition in water or methane. The use of laser spectroscopy on hydrogen isotopes was first reported by Bergamaschi et al. in 1994. They directly measured CHD/CH in atmospheric methane using a lead salt tunable diode laser spectroscopy. The development of CRDS was first reported by O'Keefe et al. in 1988. In 1999, Kerstel et al. successfully applied this technique to determine HIC in water. The system consists of a laser and a Optical cavity, cavity equipped with high finesse reflectivity mirrors. Laser light is injected into the cavity, where the resonance takes place due to the constructive interference. The laser then is turned off. The decay of light intensity is measured. In the presence of a water sample, the photo-absorption by water isotopologues follows the kinetic law. The optical spectrum is obtained by recording ring-down time of the HO spectral features of interest at certain laser wavelength. The concentration of each isotopologue is proportional to the area under each measured isotopologue spectral feature.
Laser spectroscopy is quick, simple, and relatively cheap; and the equipment is portable. So it can be used in the field for measuring water samples. H/H and O/O can be determined simultaneously from a single injection. It requires a small sample size, < 1 ö¥L for water. Typical precision is ~ 1ã¯. However, this is a compound-specific instrument, i.e. only one specific compound can be measured. And coexisting organic compounds (i.e. ethanol) could interfere with the optical light absorption features of water, resulting in cross-contamination.
SNIF-NMR
H-Site-specific Natural Isotope Fractionation-Nuclear Magnetic Resonance (Isotopic analysis by nuclear magnetic resonance, H-SNIF-NMR) is a type of NMR specialized in measuring the H concentration of organic molecules at natural abundances. The NMR spectra distinguish hydrogen atoms in different chemical environments (e.g. the order of carbon that hydrogen binds to, adjacent functional groups, and even geminal positions of methylene groups), making it a powerful tool for position-specific isotope analysis. The chemical shift (in frequency units) of H is 6.5x lower than that of H. Thus, it is hard to resolve H peaks. To provide enough resolution to separate H peaks, high-strength magnetic field instruments (~11.4T) are applied. Use of NMR to study hydrogen isotopes of natural products, was pioneered by Gerard Martin and his co-workers in the 1980s. For several decades it has been developed and expanded. The D/H NMR measurement is sometimes coupled with IR-MS measurement to create a referential standard. The sensitivity of SNIF-NMR is relatively low, typically requiring ~1 mmol of samples for each measurement. The precision with respect to isotope ratio is also poor compared to mass spectrometry. Even state-of-art instruments can only measure DHR with around 50~200㯠error depending on the compound. Therefore, so far technique can only distinguish the large D/H variations in preserved materials. In 2007, Philippe Lesot and his colleagues advanced this technique with a Two-dimensional nuclear magnetic resonance spectroscopy, 2-dimensional NMR using chiral liquid crystals (CLC) instead of isotropic solvents to dissolve organic molecules. This enables the measurements of quadrupolar doublets for each nonequivalent deuterium atom. Thus reduces peak overlaps and provides more detailed information of hydrogen chemical environment. Mainstream Isotopic analysis by nuclear magnetic resonance#Examples of applications of 2H-SNIF-NMR, uses of H-SNIF-NMR have been in source attribution, forensics andIntact molecular isotope ratio mass spectrometry
Conventionally, mass spectrometry, such as gas chromatography-mass spectrometry (GC-MS) and gas chromatography -time of flight(Time-of-flight mass spectrometry, GC-TOF), is a common technique for analyzing Isotopic labeling, isotopically labeled molecules. This method involves ionizing and analyzing isotopologues of an intact organic molecule of interest rather than its products of pyrolysis or conversion. However, it does not work for natural abundance hydrogen isotopes because conventional mass spectrometers do not have enough Resolution (mass spectrometry), mass-resolving power to measure the C/DHydrologic cycle
Isotope fractionation in the water cycle
 Water is the main source of hydrogen for all living things, so the isotopic composition of environmental water is a first-order control on that of the biosphere. The water (hydrological) cycle moves water around Earth's surface, significantly fractionating the hydrogen isotopes in water. As the atmosphere's main moisture source, the ocean has a fairly uniform HIC across the globe around 0㯠(VSMOW). Variations of öÇD larger than 10㯠in the ocean are generally confined to surface water due to evaporation, sea ice formation, and addition of meteoric water by precipitation, rivers or icebergs. In the water cycle, the two main processes that fractionate hydrogen isotopes from seawater are evaporation and condensation. Oxygen isotopic composition (O/O) of water is also an important tracer in the water cycle, and cannot be separated from hydrogen isotopes when we talk about isotope fractionation processes associated with water.
When water evaporates from the ocean to the air, both equilibrium and kinetic isotope effects occur to determine the hydrogen and oxygen isotopic composition of the resulting water vapor. At the water-air interface, a stagnant boundary layer is saturated with water vapor (100% relative humidity), and the isotopic composition of water vapor in the boundary layer reflects an equilibrium fractionation with liquid water. The liquid-vapor equilibrium fractionations for hydrogen and oxygen isotopes are temperature-dependent:
: (ã¯)
: (ã¯)
The amount of liquid-vapor equilibrium fractionation for hydrogen isotopes is about 8x that of oxygen isotopes at Earth surface temperatures, which reflects the relative mass differences of the two isotope systems: H is 100% heavier than H, O is 12.5% heavier than O. Above the boundary layer, there is a transition zone with relative humidity less than 100%, and there is a kinetic isotope fractionation associated with water vapor diffusion from the boundary layer to the transition zone, which is empirically related to the relative humidity (h):
: ã¯
: ã¯
The KIE associated with diffusion reflects the mass difference of the heavy-isotope water molecules HHO and HO relative to the normal isotopolog (HO).
After water evaporates to the air, condensation and precipitation transport it and return it to the surface. Water vapor condenses in ascending air masses that develop a lower temperature and saturation vapor pressure. Since the cooling and condensation happen relatively slowly, it is a process with equilibrium isotope effects. However, as water vapor is progressively condensed and lost from the air during moisture transport, the isotopic composition of the remaining vapor, as well as the resulting precipitation, can be largely depleted due to the process of Rayleigh fractionation, Rayleigh distillation. The equation for Rayleigh distillation is:
Water is the main source of hydrogen for all living things, so the isotopic composition of environmental water is a first-order control on that of the biosphere. The water (hydrological) cycle moves water around Earth's surface, significantly fractionating the hydrogen isotopes in water. As the atmosphere's main moisture source, the ocean has a fairly uniform HIC across the globe around 0㯠(VSMOW). Variations of öÇD larger than 10㯠in the ocean are generally confined to surface water due to evaporation, sea ice formation, and addition of meteoric water by precipitation, rivers or icebergs. In the water cycle, the two main processes that fractionate hydrogen isotopes from seawater are evaporation and condensation. Oxygen isotopic composition (O/O) of water is also an important tracer in the water cycle, and cannot be separated from hydrogen isotopes when we talk about isotope fractionation processes associated with water.
When water evaporates from the ocean to the air, both equilibrium and kinetic isotope effects occur to determine the hydrogen and oxygen isotopic composition of the resulting water vapor. At the water-air interface, a stagnant boundary layer is saturated with water vapor (100% relative humidity), and the isotopic composition of water vapor in the boundary layer reflects an equilibrium fractionation with liquid water. The liquid-vapor equilibrium fractionations for hydrogen and oxygen isotopes are temperature-dependent:
: (ã¯)
: (ã¯)
The amount of liquid-vapor equilibrium fractionation for hydrogen isotopes is about 8x that of oxygen isotopes at Earth surface temperatures, which reflects the relative mass differences of the two isotope systems: H is 100% heavier than H, O is 12.5% heavier than O. Above the boundary layer, there is a transition zone with relative humidity less than 100%, and there is a kinetic isotope fractionation associated with water vapor diffusion from the boundary layer to the transition zone, which is empirically related to the relative humidity (h):
: ã¯
: ã¯
The KIE associated with diffusion reflects the mass difference of the heavy-isotope water molecules HHO and HO relative to the normal isotopolog (HO).
After water evaporates to the air, condensation and precipitation transport it and return it to the surface. Water vapor condenses in ascending air masses that develop a lower temperature and saturation vapor pressure. Since the cooling and condensation happen relatively slowly, it is a process with equilibrium isotope effects. However, as water vapor is progressively condensed and lost from the air during moisture transport, the isotopic composition of the remaining vapor, as well as the resulting precipitation, can be largely depleted due to the process of Rayleigh fractionation, Rayleigh distillation. The equation for Rayleigh distillation is:
 where R is the isotope ratio in the initial water vapor, R is the isotope ratio in the remaining water vapor after some condensation, f is the fraction of water vapor remaining in the air, and öÝ is the liquid-vapor equilibrium fractionation factor (öÝ=1+öç). The isotopic composition of the resulting precipitation (R) can be derived from the composition of the remaining vapor:
As f decreases progressively during condensation, the remaining vapor becomes more and more depleted of the heavy isotopes, and the depletion becomes larger as f approaches zero. Rayleigh distillation can explain some first-order spatial patterns observed in the isotopic composition of precipitation across the globe, including isotopic depletion from the tropics to the poles, isotopic depletion from coastal to inland regions, and isotopic depletion with elevation over a mountain rang
where R is the isotope ratio in the initial water vapor, R is the isotope ratio in the remaining water vapor after some condensation, f is the fraction of water vapor remaining in the air, and öÝ is the liquid-vapor equilibrium fractionation factor (öÝ=1+öç). The isotopic composition of the resulting precipitation (R) can be derived from the composition of the remaining vapor:
As f decreases progressively during condensation, the remaining vapor becomes more and more depleted of the heavy isotopes, and the depletion becomes larger as f approaches zero. Rayleigh distillation can explain some first-order spatial patterns observed in the isotopic composition of precipitation across the globe, including isotopic depletion from the tropics to the poles, isotopic depletion from coastal to inland regions, and isotopic depletion with elevation over a mountain rangall of which are associated with progressive moisture loss during transport. The Rayleigh distillation model can also be used to explain the strong correlation between öÇD and öÇO in global precipitation, expressed as the
Water isotopes and climate
Based on the processes that fractionate isotopes in the water cycle, isotopic composition of meteoric water can be used to infer related environmental variables such as air temperature, precipitation amount, past elevations, lake levels, as well as to trace moisture sources. These studies form the field of isotope hydrology. Examples of isotope hydrology applications include:Temperature reconstruction
Isotopic composition of precipitation can be used to infer changes in air temperature based on the Rayleigh process. Lower temperature corresponds to lower saturation vapor pressure, which leads to more condensation that drives the residual vapor toward isotope depletion. The resulting precipitation thus has a more negative öÇD and öÇO value at lower temperature. This precipitation isotope thermometer is more sensitive at lower temperatures, and widely applied at high latitudes. For example, öÇD and öÇO were found to have a temperature sensitivity of 8ã¯/ô¯C and 0.9ã¯/ô¯C in Antarctic snow, and a sensitivity of 5.6ã¯/ô¯C and 0.69ã¯/ô¯C across Arctic sites. öÇD and öÇO of ice cores in Greenland, Antarctica and alpine glaciers are important archives of temperature change in the geological past.Precipitation amount effect
In contrast to temperature control at high latitudes, the isotopic composition of precipitation in the tropics is mainly influenced by rainfall amount (negative correlation). This "amount effect" is also observed for summer precipitation in the subtropics. Willi Dansgaard, who first proposed the term "amount effect", suggested several possible reasons for the correlation: (1) As cooling and condensation progress, the rainfall isotopic composition reflects an integrated isotopic depletion by the Rayleigh process; (2) A small amount of rainfall is more likely to be influenced by evaporation and exchange with surrounding moisture, which tend to make it more isotopically enriched. At low latitudes, the amount effect for öÇO is around ã1.6ã¯/100mm precipitation increase at island stations, and ã2.0ã¯/100mm at continental stations. It was also noted that the amount effect was most pronounced when comparing isotopic composition of monthly precipitation at different places in the tropics. The amount effect is also expected for HIC, but there are not as many calibration studies. Across southeast Asia, the öÇD sensitivity to monthly precipitation amount varies between ã15 and ã25ã¯/100mm depending on location. In temperate regions, the isotopic composition of precipitation is dominated by rainfall amount in summer, but more controlled by temperature in the winter. The amount effect may also be complicated by changes in regional moisture sources. Reconstructions of rainfall amount in the tropics in the geological past are mostly based on öÇO of speleothems or öÇD of biogenic lipids, both of which are thought of as proxies for the isotopic composition of precipitation.Applications
Isotope hydrology
Hydrogen and oxygen isotopes also work as tracers for water budget in terrestrial reservoirs, including lakes, rivers, groundwater and soil water. For a lake, both the amount of water in the lake and the isotopic composition of the water are determined by a balance between inputs (precipitation, stream and ground water inflow) and outputs (evaporation, stream and ground water outflow). The isotopic composition of lake water can often be used to track evaporation, which causes isotope enrichment in the lake water, as well as a öÇD-öÇO slope that is shallower than the meteoric water line. The isotopic composition of river water is highly variable and have complicated sources over different timescales, but can generally be treated as a two-endmember mixing problem, a base-flow endmember (mainly ground water recharge) and an overland-flow endmember (mainly storm events). The isotope data suggest that the long-term integrated base-flow endmember is more important in most rivers, even during peak flows in summer. Systematic river isotope data were collected across the world by the Global Network of Isotopes in Rivers (GNI.The isotopic composition of groundwater can also be used to trace its sources and flow paths. An example is a groundwater isotope mapping study in Sacramento, California, which showed lateral flow of river water with a distinct isotope composition into the groundwater that developed a significant water table depression due to pumping for human use. The same study also showed an isotopic signal of agricultural water being recharged into the giant alluvial aquifer in California's Central Valley. Finally, the isotopic composition of soil water is important for the study of plants. Below the water table, the soil has a relatively constant source of water with a certain isotopic composition. Above the water table, the isotopic composition of soil water is enriched by evaporation until a maximum at the surface. The vertical profile of isotopic composition of soil water is maintained by the diffusion of both liquid and vapor water. A comparison of soil water and plant xylem water öÇD can be used to infer the depth at which plant roots get water from the soil.
Paleo-reconstruction
Ice core records
 The isotopic compositions of ice cores from continental ice sheets and alpine glaciers have been developed as temperature proxies since the 1950s. Samuel Epstein was one of the first to show the applicability of this proxy by measuring oxygen isotopes in Antarctic snow, and also pointed out complications in the stable isotope-temperature correlation caused by the history of the air masses from which the snow formed. Ice cores in Greenland and Antarctica can be thousands of meters thick and record snow isotopic composition of the past few glacial-interglacial cycles. Ice cores can be dated by layer counting on the top and ice flow modeling at depth, with additional age constraints from volcanic ash. Cores from Greenland and Antarctica can be aligned in age at high-resolution by comparing globally well-mixed trace gas (e.g. CH) concentrations in the air bubbles trapped in the cores. Some of the first ice core records from Greenland and Antarctica with age estimates go back to the last 10 years, and showed a depletion in öÇD and öÇO in the last ice age. The ice core record has since been extended to the last 800,000 years in Antarctica, and at least 250,000 years in Greenland. One of the best öÇD-based ice core temperature records is from the Vostok ice core in Antarctica, which goes back to 420,000 years. The öÇD-temperature (of the Inversion (meteorology), inversion layer where snow forms) conversion in east Antarctica based on modern spatial gradient of öÇD (9ã¯/ô¯C) is öT=(ööÇD-8ööÇO)/9, which takes into account variations in seawater isotopic composition caused by global ice volume changes. Many local effects can influence ice öÇD in addition to temperature. These effects include moisture origin and transport pathways, evaporation conditions and precipitation seasonality, which can be accounted for in more complicated models. Nevertheless, the Vostok ice core record shows some very important results: (1) A consistent öÇD depletion of ~70㯠during the last four glacial periods compared to interglacial times, corresponding to a cooling of 8ô¯C in Antarctica; (2) A consistent drop of atmospheric CO concentration by 100 ppmv and CH drop by ~300 ppbv during glacial times relative to interglacials, suggesting a role of greenhouse gases in regulating global climate; (3) Antarctic air temperature and greenhouse gas concentration changes precede global ice volume and Greenland air temperature changes during glacial terminations, and greenhouse gases may be an amplifier of insolation forcing during glacial-interglacial cycles. Greenland ice core isotope records, in addition to showing glacial-interglacial cycles, also shows millennial-scale climate oscillations that may reflect reorganization in ocean circulation caused by ice melt charges. There have also been ice core records generated in alpine glacials on different continents. A record from the Andes Mountains in Peru shows a temperature decrease of 5-6ô¯C in the tropics during the last ice age. A record from the Tibetan plateau shows a similar isotope shift and cooling during the last ice age. Other existing alpine glacial isotope records include Mount Kilimanjaro in Tanzania, Mount Altai and West Belukha Plateau in Russia, Mount Logan in Canada, the Fremont Glacier in Wyoming, USA, and the Illimani Ice Core in Bolivia, most of which cover an interval of the Holocene epoc
The isotopic compositions of ice cores from continental ice sheets and alpine glaciers have been developed as temperature proxies since the 1950s. Samuel Epstein was one of the first to show the applicability of this proxy by measuring oxygen isotopes in Antarctic snow, and also pointed out complications in the stable isotope-temperature correlation caused by the history of the air masses from which the snow formed. Ice cores in Greenland and Antarctica can be thousands of meters thick and record snow isotopic composition of the past few glacial-interglacial cycles. Ice cores can be dated by layer counting on the top and ice flow modeling at depth, with additional age constraints from volcanic ash. Cores from Greenland and Antarctica can be aligned in age at high-resolution by comparing globally well-mixed trace gas (e.g. CH) concentrations in the air bubbles trapped in the cores. Some of the first ice core records from Greenland and Antarctica with age estimates go back to the last 10 years, and showed a depletion in öÇD and öÇO in the last ice age. The ice core record has since been extended to the last 800,000 years in Antarctica, and at least 250,000 years in Greenland. One of the best öÇD-based ice core temperature records is from the Vostok ice core in Antarctica, which goes back to 420,000 years. The öÇD-temperature (of the Inversion (meteorology), inversion layer where snow forms) conversion in east Antarctica based on modern spatial gradient of öÇD (9ã¯/ô¯C) is öT=(ööÇD-8ööÇO)/9, which takes into account variations in seawater isotopic composition caused by global ice volume changes. Many local effects can influence ice öÇD in addition to temperature. These effects include moisture origin and transport pathways, evaporation conditions and precipitation seasonality, which can be accounted for in more complicated models. Nevertheless, the Vostok ice core record shows some very important results: (1) A consistent öÇD depletion of ~70㯠during the last four glacial periods compared to interglacial times, corresponding to a cooling of 8ô¯C in Antarctica; (2) A consistent drop of atmospheric CO concentration by 100 ppmv and CH drop by ~300 ppbv during glacial times relative to interglacials, suggesting a role of greenhouse gases in regulating global climate; (3) Antarctic air temperature and greenhouse gas concentration changes precede global ice volume and Greenland air temperature changes during glacial terminations, and greenhouse gases may be an amplifier of insolation forcing during glacial-interglacial cycles. Greenland ice core isotope records, in addition to showing glacial-interglacial cycles, also shows millennial-scale climate oscillations that may reflect reorganization in ocean circulation caused by ice melt charges. There have also been ice core records generated in alpine glacials on different continents. A record from the Andes Mountains in Peru shows a temperature decrease of 5-6ô¯C in the tropics during the last ice age. A record from the Tibetan plateau shows a similar isotope shift and cooling during the last ice age. Other existing alpine glacial isotope records include Mount Kilimanjaro in Tanzania, Mount Altai and West Belukha Plateau in Russia, Mount Logan in Canada, the Fremont Glacier in Wyoming, USA, and the Illimani Ice Core in Bolivia, most of which cover an interval of the Holocene epocBiomolecules
The isotopic composition of biomolecules preserved in the sedimentary record can be used as a proxy (climate), proxy for paleoenvironment reconstructions. Since water is the main hydrogen source for Phototroph, photoautotrophs, the HIC of their biomass can be related to the composition of their growth water and thereby used to gain insight into some properties of ancient environments. Studying hydrogen isotopes can be very valuable, as hydrogen is more directly related to climate than other relevant stable isotope systems. However, hydrogen atoms bonded to oxygen, nitrogen, or sulfur are exchangeable with environmental hydrogen, which makes this system less straightforward [ref to earlier H exchange section]. To study the HIC of biomolecules, it is preferable to use compounds where the hydrogen is largely bound to carbon, and thus not exchangeable on experimental timescales. By this criterion,= Cellulose
= The carbon-bonded HIC of= Plant leaf waxes
= Terrestrial plants make Epicuticular wax, leaf waxes to coat the surfaces of their leaves, to minimize water loss. These waxes are largely straight-chain ''n''-alkyl lipids. They are insoluble, non-volatile, chemically inert, and resistant to degradation, making them easily preserved in the sedimentary record, and therefore good targets as biomarkers.
The main water source for land plants is soil water, which largely resembles the HIC of rain water, but varies between environments and with enrichment by precipitation, depletion by evaporation, and exchange with atmospheric water vapor. There can be a significant offset between the öÇD value of source water and the öÇD value of leaf water at the site of lipid biosynthesis. No fractionation is associated with water uptake by roots, a process usually driven by capillary tension, with the one exception of xerophytes that burn ATP to pump water in extremely arid environments (with a roughly 10㯠depletion). However, leaf water can be substantially enriched relative to soil water due to transpiration, an evaporative process which is influenced by temperature, humidity, and the composition of surrounding water vapor. The leaf water HIC can be described with a modified Craig-Gordon model, where öD is the steady state enrichment of leaf water, öç is the temperature-dependent equilibrium fractionation between liquid water and vapor, öç is the KIE from diffusion between leaf internal air space and the atmosphere, öD is the leaf/air disequilibrium, ''e'' is atmospheric vapor pressure, and ''e'' is internal leaf vapor pressure.
:
The Pûˋclet number, Pûˋclet effect, which describes the opposing forces of advection and diffusion can be added to the model as
:
where E is transpiration rate, L is length scale of transport, C is concentration of water, and D is diffusion coefficient.
:
While the role of rain water öÇD as the fundamental control on the final öÇD of lipids is well documented, the importance of fractionation effects from rain water to soil water and leaf water on öç is appreciated but remains poorly understood.
Organic biomolecules are generally depleted relative to the öÇD of leaf water. However, differences between organisms, biosynthetic pathways, and biological roles of different molecules can lead to huge variability in fractionation; the diversity of lipid biomarkers spans a 600㯠range of öÇD values. Lipid biosynthesis is biochemically complex, involving multiple enzyme-dependent steps that can lead to isotope fractionations. There are three major pathways of lipid biosynthesis, known as the mevalonate pathway, Fatty acid synthesis, the acetogenic pathway, and Non-mevalonate pathway, the 1-deoxyD-xylulose-5-phosphate/2-methylerythroyl-4-phosphate pathway. The acetogenic pathway is responsible for the production of ''n''-alkyl lipids like leaf waxes, and is associated with a smaller öÇD depletion relative to source water than the other two lipid biosynthesis pathways. While leaf water is the main source of hydrogen in leaf biomolecules, relatively depleted hydrogen from acetate or NADPH is often added during biosynthesis, and contributes to the HIC of the final molecule. Secondary hydrogen exchange reactions, meaning hydrogenation and dehydrogenation reactions outside of the primary biosynthetic pathway, also contribute substantially to the variability of lipid HIC.
It is important to note that biological differences in fractionation stem not only from biochemical differences between different molecules, but also from physiological differences between different organisms. For example, the öÇDs of multiple leaf wax molecules are enriched in shrubs (median ~ ã90ã¯) relative to trees (median ~ ã135ã¯), which themselves are enriched relative to both C3 carbon fixation, (median ~ ã160ã¯) and C4 carbon fixation, grasses (median ~ ã140ã¯). Between individual species, substantial variation in öÇD has been documented. Other physiological factors that contribute to variable leaf wax öÇD values include the seasonal timing of leaf development, response to external stress or environmental variability, and the presence or absence of stomata
It can be difficult to distinguish between physiological factors and environmental factors, when many physiological adaptations are directly related to environment.
Several environmental factors have been shown to contribute to leaf wax öÇD variability, in addition to environmental effects on the öÇD of source water. Humidity is known to impact lipid öÇD at moderate humidity levels, but not at particularly high (>80%) or low (<40%) humidity levels, and a broad trend of enriched öÇDs, meaning smaller öç, is seen in arid regions. Temperature and sunlight intensity, both correlated to latitude, have strong effects on the rates of metabolism and transpiration, and by extension on öç. Also, the average chain length of leaf wax molecules varies with geographic latitude, and öç has been shown to increase with increasing chain length.
When using biomarkers as a proxy for reconstructing ancient environments, it is important to be aware of the biases inherent in the sedimentary record. Leaf matter incorporated into sediment is largely deposited in the autumn, so seasonal variations in leaf waxes must be considered accordingly. Furthermore, sediments average leaf waxes over lots of different plants in both space and time, making it difficult to calibrate the biological constraints on öç. Finally, preservation of biomolecules in the geologic record does not faithfully represent whole ecosystems, and there is always the threat of hydrogen exchange, particularly if the sediments are subjected to high temperatures.
The HIC of leaf waxes can be summarized as the öÇD of rain water, with three main fractionation steps: evaporation from soil water, transpiration from leaf water, and lipid biosynthesis, which can be combined and measured as the net fractionation, öç. With ever-improving measurement techniques for single molecules, and correlation with other independent proxies in the geological record that can help constrain some variables, investigating the HIC of leaf waxes can be extremely productive. Leaf wax öÇD data has been successfully applied to improving our understanding of climate driven changes in terrestrial hydrology, by showing that ocean circulation and surface temperature have a significant effect on continental precipitation. Leaf wax öÇD values have also been used as records of paleoaltimetry to reconstruct the elevation gradients in ancient mountain ranges based on the effect of altitude on rain water öÇD.
Terrestrial plants make Epicuticular wax, leaf waxes to coat the surfaces of their leaves, to minimize water loss. These waxes are largely straight-chain ''n''-alkyl lipids. They are insoluble, non-volatile, chemically inert, and resistant to degradation, making them easily preserved in the sedimentary record, and therefore good targets as biomarkers.
The main water source for land plants is soil water, which largely resembles the HIC of rain water, but varies between environments and with enrichment by precipitation, depletion by evaporation, and exchange with atmospheric water vapor. There can be a significant offset between the öÇD value of source water and the öÇD value of leaf water at the site of lipid biosynthesis. No fractionation is associated with water uptake by roots, a process usually driven by capillary tension, with the one exception of xerophytes that burn ATP to pump water in extremely arid environments (with a roughly 10㯠depletion). However, leaf water can be substantially enriched relative to soil water due to transpiration, an evaporative process which is influenced by temperature, humidity, and the composition of surrounding water vapor. The leaf water HIC can be described with a modified Craig-Gordon model, where öD is the steady state enrichment of leaf water, öç is the temperature-dependent equilibrium fractionation between liquid water and vapor, öç is the KIE from diffusion between leaf internal air space and the atmosphere, öD is the leaf/air disequilibrium, ''e'' is atmospheric vapor pressure, and ''e'' is internal leaf vapor pressure.
:
The Pûˋclet number, Pûˋclet effect, which describes the opposing forces of advection and diffusion can be added to the model as
:
where E is transpiration rate, L is length scale of transport, C is concentration of water, and D is diffusion coefficient.
:
While the role of rain water öÇD as the fundamental control on the final öÇD of lipids is well documented, the importance of fractionation effects from rain water to soil water and leaf water on öç is appreciated but remains poorly understood.
Organic biomolecules are generally depleted relative to the öÇD of leaf water. However, differences between organisms, biosynthetic pathways, and biological roles of different molecules can lead to huge variability in fractionation; the diversity of lipid biomarkers spans a 600㯠range of öÇD values. Lipid biosynthesis is biochemically complex, involving multiple enzyme-dependent steps that can lead to isotope fractionations. There are three major pathways of lipid biosynthesis, known as the mevalonate pathway, Fatty acid synthesis, the acetogenic pathway, and Non-mevalonate pathway, the 1-deoxyD-xylulose-5-phosphate/2-methylerythroyl-4-phosphate pathway. The acetogenic pathway is responsible for the production of ''n''-alkyl lipids like leaf waxes, and is associated with a smaller öÇD depletion relative to source water than the other two lipid biosynthesis pathways. While leaf water is the main source of hydrogen in leaf biomolecules, relatively depleted hydrogen from acetate or NADPH is often added during biosynthesis, and contributes to the HIC of the final molecule. Secondary hydrogen exchange reactions, meaning hydrogenation and dehydrogenation reactions outside of the primary biosynthetic pathway, also contribute substantially to the variability of lipid HIC.
It is important to note that biological differences in fractionation stem not only from biochemical differences between different molecules, but also from physiological differences between different organisms. For example, the öÇDs of multiple leaf wax molecules are enriched in shrubs (median ~ ã90ã¯) relative to trees (median ~ ã135ã¯), which themselves are enriched relative to both C3 carbon fixation, (median ~ ã160ã¯) and C4 carbon fixation, grasses (median ~ ã140ã¯). Between individual species, substantial variation in öÇD has been documented. Other physiological factors that contribute to variable leaf wax öÇD values include the seasonal timing of leaf development, response to external stress or environmental variability, and the presence or absence of stomata
It can be difficult to distinguish between physiological factors and environmental factors, when many physiological adaptations are directly related to environment.
Several environmental factors have been shown to contribute to leaf wax öÇD variability, in addition to environmental effects on the öÇD of source water. Humidity is known to impact lipid öÇD at moderate humidity levels, but not at particularly high (>80%) or low (<40%) humidity levels, and a broad trend of enriched öÇDs, meaning smaller öç, is seen in arid regions. Temperature and sunlight intensity, both correlated to latitude, have strong effects on the rates of metabolism and transpiration, and by extension on öç. Also, the average chain length of leaf wax molecules varies with geographic latitude, and öç has been shown to increase with increasing chain length.
When using biomarkers as a proxy for reconstructing ancient environments, it is important to be aware of the biases inherent in the sedimentary record. Leaf matter incorporated into sediment is largely deposited in the autumn, so seasonal variations in leaf waxes must be considered accordingly. Furthermore, sediments average leaf waxes over lots of different plants in both space and time, making it difficult to calibrate the biological constraints on öç. Finally, preservation of biomolecules in the geologic record does not faithfully represent whole ecosystems, and there is always the threat of hydrogen exchange, particularly if the sediments are subjected to high temperatures.
The HIC of leaf waxes can be summarized as the öÇD of rain water, with three main fractionation steps: evaporation from soil water, transpiration from leaf water, and lipid biosynthesis, which can be combined and measured as the net fractionation, öç. With ever-improving measurement techniques for single molecules, and correlation with other independent proxies in the geological record that can help constrain some variables, investigating the HIC of leaf waxes can be extremely productive. Leaf wax öÇD data has been successfully applied to improving our understanding of climate driven changes in terrestrial hydrology, by showing that ocean circulation and surface temperature have a significant effect on continental precipitation. Leaf wax öÇD values have also been used as records of paleoaltimetry to reconstruct the elevation gradients in ancient mountain ranges based on the effect of altitude on rain water öÇD.
= Alkenones
= Another group of molecules often used in paleoreconstructions are alkenones, long-chain largely unsaturated lipids produced exclusively by coccolithophores. Coccolithophores are marine haptophyte algae, and include the globally iconic species ''Emiliania huxleyi'', one of the main Calcium carbonate, CaCO producers in the ocean. The öÇDs of alkenones are highly correlated to the öÇDs of seawater, and so can be used to reconstruct paleoenvironmental properties that constrain the isotopic composition of sea water. The most notable reconstruction that alkenone öÇD values are applied to is the salinity of ancient oceans. Both the öÇDs of sea water and the fractionations associated with hyptophyte biochemistry (öç) are fairly well understood, so alkenones can be readily used to observe the secondary effect of salinity on öÇD. There is a well established positive linear correlation between salinity and öç, on the order of a ~3㯠change in fractionation per salinity unit. Hypothesized mechanisms for this effect include enrichment of D in intracellular water due to reduced exchange with extracellular water at higher salinity, removal of H from intracellular water due to increased production of solutes to maintain osmotic pressure at higher salinity, and lower haptophyte growth rates at higher salinity Alkenone öÇDs have been used successfully to reconstruct past salinity changes in the Mediterranean Sea, Black Sea, Panama Basin, and Mozambique Channel. As an extension of salinity, this data was also used to draw further conclusions about ancient environments, such as ancient freshwater flooding events, and the evolution of plankton in response to environmental changesStable isotope paleoaltimetry
The possibility of using water isotope depletion with elevation to reconstruct paleoaltimetry was demonstrated as early as the late 1960s, when Caltech geochemist Samuel Epstein tried to collect rainwater at different elevations in a single storm. The öÇO and öÇD lapse rates vary within -1 to -5ã¯/km and -10 to -40ã¯/km respectively, but can vary with locations and seasons, and are not exactly linear with altitude. One of the first studies in stable isotope paleoaltimetry demonstrated a meteoric water öÇD signature of -90 to -139㯠in fluid inclusions in quartz and adularia in an epithermal gold-silver deposit in Nevada, and suggested the applicability of stable isotopes in reconstruction of ancient topography in Basin and Range Province, the Great Basin. The hydrogen and oxygen isotopes of hydrous silicate minerals have since then been used to reconstruct topographic histories in mountain ranges across the world, including the North American Cordillera, the Rocky Mountains, the Himalayas, the European Alps, and Southern Alps in New Zealand. Lab experiments with clay minerals have shown that the hydrogen and oxygen isotope compositions are relatively resistant to alteration at moderate temperature (<100ô¯C), and can preserve the original meteoric water signal. One important effect of mountain ranges on rainfall stable isotopes is the rain shadow effect, in which an isotopic depletion happens in precipitation on the leeward side compared to the windward side. A change in the difference in isotopic composition of precipitation on the two sides of a mountain can be used to infer the magnitude of the rain shadow effect. In one such study, an isotope enrichment was observed in smectite on the east side of the Sierra Nevada in California from mid-Miocene to late Pliocene, suggesting a decrease in elevation during this period. Another study found öÇDs around ã140㯠in muscovite in the North America Cordillera during the early Eocene, which would suggest an elevation 1km higher than today at the time. In addition to hydrous minerals, hydrogen isotopes in biomarkers such as leaf waxes have also been developed for paleoaltimetry studies. The öÇD lapse rate in leaf waxes (ã21ã¯/km) falls in the range of meteoric water observations. As an example study, leaf wax öÇD data has been used to confirm hydrous mineral paleoaltimetry for the high elevation of the Sierra Nevada during the Eocene.Fossil fuels
The HIC of Petroleum, oil, Natural gas, gas andKerogens and coals
The first stage that sedimentary organic matter (SOM) experiences after Deposition (geology), deposition is diagenesis. During diagenesis, biological decomposition can alter the DHR of organics. Several experimental studies have shown that some biodegraded materials become slightly enriched in D (less than 50ã¯). Most organics become kerogen by the end of diagenesis. Generally, öÇD of kerogen spans a wide range. Many factors contribute to the kerogen we observe in geologic records, including: # Source water hydrogen isotope patterns: For example, lake systems are more sensitive to hydrologic cycles than marine environments. # Differential fractionation for various organisms and metabolic pathways: differences in organic composition can also reflect in primary signal. # Isotopic exchange, H loss and H addition: This can involve mixing water-derived D with the primary signal. # Generation of bitumen, oil and gas: There's a fractionation between the product and kerogen. Research on the Australian basins showed that öÇD of lacustrine algal sourced kerogen with terrestrial contributions varies from ã105㯠to ã200ã¯, and öÇD of kerogen from near-coastal depositional environment has a narrower range, ã75㯠to ã120ã¯. The smaller span in DHRs of coastal kerogen is thought to reflect the relatively stable regional climate. Pedentchouk and his colleagues reported öÇD values of -70㯠to -120㯠in immature to low mature kerogen from early Cretaceous lacustrine sediments in West Africa. Coals are from type III kerogen mostly derived from land plants, which should have a primary D/H signal sensitive to local meteoric water. Reddings et al. analyzed coals of various origins and found them randomly scattered across the range of ã90㯠to ã170ã¯. Rigby et al. found D contents decrease from ã70㯠to ã100㯠with increasing maturity in coal from Bass Basin and attributed this to latter exchange with low D water. Smith et al. studied H isotopes of coal samples from Antarctica and Australia. They found a strong negative correlation between öÇD and inferred paleolatitude. For coal samples originating near the Equator, öÇD is around ã50ã¯, while for those originating from polar regions, öÇD is around ã150ã¯. This öÇD trend along latitude is consistent meteoric water trend and thus is an evidence that coals can preserve much of the original signals. There are two types of approach to study the alteration of DHRs of kerogen during catagenesis: (1) laboratory incubation of organic matter that enables mechanistic study with controlled experiments; (2) natural sample measurement that provides information of combined effects over geologic timescales. The complex composition and chemistry of kerogen complicates the results. Nevertheless, most research on HIC of kerogen show D enrichment with increasing maturity. Type II kerogen (marine derived) from New Albany Shale is reported to have öÇD rise from ã120㯠to ã70㯠as vitrinite reflectance increase from 0.3% to 1.5%. Two main mechanisms have been proposed for enrichment. One of them is kinetic fractionation during hydrocarbon generation while the other is isotopic exchange with surrounding water. Anhydrous incubation experiments have shown that the products are generally more D-depleted than their precursors, causing enrichment in residual kerogen. Schimmelmann et al. studied the relationship between terrestrially-derived oil and their source rock kerogens from four Australian Basins. They found that on average the oil is depleted to corresponding kerogen by 23ã¯. Hydrous incubation experiments suggest that 36ã79% of bulk organic hydrogen may come from water at moderate maturity. While still under debate, it appears likely that incorporation of water hydrogen isotopes is the more dominant process for kerogen D- enrichment during catagenesis. In summary, D content of kerogen and coal is complicated and hard to resolve due to the complex chemistry. Nevertheless, studies have found the possible correlation between coal öÇD and paleo-latitude.Natural gas
Commonly, HIC of natural gas from the same well has a trend of öÇD < öÇD < öÇD < öÇD. This is because most natural gas is thought to be generated by stepwise Cracking (chemistry), thermal cracking that is mostly irreversible and thus governed by normalOil
Oil is generally a product of thermal breakdown of type I and type II kerogen during Catagenesis (geology), catagenesis. The HIC should reflect the source kerogen signal, generation fractionation, isotopic exchange and other maturation effects. Thermal maturation at the oil window can erase much of the HIC primary signals. The formation of oil involves breaking C-C and C-H bonds, resulting in depletion of C and H in the products and enrichment in the residual reactants due to KIEs. Yongchun Tang and his colleagues modeled this process based on laboratory-calibrated kinetics data and found that the Pre-exponential factor, frequency factor ratio for D/H is 1.07. Moreover, oil is also affected by isotope fractionation from phase transition, phase changes. However, the behavior of oil gas-liquid fractionation differs from water as the vapor phase of oil is H-enriched. This depletes residual oil as it gets evaporated. Biodegradation of oil is also expected to fractionate hydrogen isotopes, as enzymatic breaking of C-H bond has a normal KIE. Several degradation experiments show that this fractionation is generally mild, ranging from ã11㯠to ã79ã¯. This process should also enrich partially degraded oil. Finally, oil stored in a reservoir often had migrated through subsurface (aka geochromatography) from another source region, interacting with water. No data has been published to confirm the fractionation associated with migration, yet theoretical prediction shows that this is likely to be very small. Many studies of natural samples have shown slight increases in öÇD with thermal maturity. Amane Waseda reported öÇD of oil samples in northeast Japan to increase from around ã130㯠to around ã110㯠with higher maturity. At low thermal maturity, dos Santos Neto and Hayes reported öÇD of Saturated fat, saturate fraction of oil in Portiguar Basin derived from a lacustrine environment is -90ã¯, and from a marine-evaporitic environment is -120㯠to ã135ã¯. Bulk analysis of oil, which yields a complex mixture of organic compounds, obscures much of the valuable information. Switching to compound-specific study greatly expanded our understanding of hydrogen isotopes of oil. Analyzing HIC at the compound level avoids problems from differences in exchange rates, simplifies sources and products relationships, and draws a much more detailed picture. öÇDs of ''n''-alkanes are generally thought to be representative of oil as they are the major components. Schimmelmann et al. confirmed that alkane fractions have almost the same DHRs as Crude oil, whole oils. Depending on source material type and maturity, öÇD of ''n''-alkanes can vary from ã100㯠to ã180ã¯. A common phenomenon of various oil and matured rock derived n-alkanes is a trend of increasing öÇD with chain length. For example, Li et al. analyzed oils from the Western Canada Sedimentary Basin and found öÇD increased between 20㯠and 40㯠from C to C. This "isotope slope" is an artifact of kinetic fractionation associated with thermal cracking of carbon chains. This trend has been experimentally reproduced and theoretically modeled by Tang et al. N-alkanes are also known to preserve detailed information of source material. Li et al. studied oils from the marine-derived Upper Cretaceous Second White Speckled Shale and found strong depleted signal around ã180㯠in C-C. The low öÇD of this marine samples was explained by the discharge of a large high latitude river. Schimmelmann et al. found that the öÇD of the oil sampled from coaly facies of the Crayfish group reaches down to ã230㯠where as those sampled from algal facies of the same group are around ã100ã¯. Such huge variation is hard to explain by any other causes than Australia splitting from Antarctica in late Cretaceous. Another special case reported by Xiong et al. studied Ordovician carbonates from Bohai Bay Basin. They found big differences between öÇD of n-alkanes, reflecting that the original signal is preserved rather than being homogenized. The result is not obvious as the sample is very mature (inferred vitrinite reflectance R up to 2.3). Thus this is strong evidence that carbonate systems have much lower catalytic efficiency of hydrogen exchange on hydrocarbons. Strong enrichment (~40ã¯) in odd carbon numbered alkanes to even carbon numbered alkanes is also found in some subset of samples and the reason is unclear at this point. This odd-even effect is also observed in immature Clastic rock, clastic sediments.Ecohydrology
Ecohydrology is concerned with the interaction between ecosystems and water cycling, from measuring the small scale drainage of Soil water (retention), water into soil to tracking the broad movements of water evaporating from trees. Because deuterium acts as a conservative Isotopic labeling, tracer, it works well for tracking water movement through plants and ecosystems. Though water movement in single-process phenomena such as evaporation is relatively simple to track, many systems (e.g. cloud forests) in the environment have multiple sources, and tracking water movement becomes more complicated. Isotope spiking can also be done to determine water transport through soil and into plants by injecting Heavy water, deuterated water directly into the ground. Stable isotope analysis of xylem water can be used to follow the movement of water from soil into the plants and therefore provide a record of the depth of water acquisition. An advantage to using xylem water is that in theory, the HIC should directly reflect the input water without being affected by leaf transpiration. For example, Dawson and Ehleringer used this approach to determine whether trees that grow next to streams are using the surface waters from that stream. Water from the surface would have the same isotopic composition as the stream, while water from farther below in the ground would be from past precipitation inputs. In this case, younger trees had a xylem water isotopic composition very close to the adjacent stream and likely used surface waters to get established. Older trees had depleted xylem water relative to the stream, reflecting that they source their water from deeper underground. Other stable isotope studies have also determined that plants in redwood forests do not just take up water from their roots but acquire a significant proportion of water via stomatal uptake on leaves. Plant water can be used to characterize other plant physiological processes that affect theEcology
Migration patterns
HIC can be useful in tracking animal migration. Animals with metabolically inert tissue (e.g. feathers or hair) synthesize that tissue using hydrogen from source water and food, but ideally do not incorporate subsequent water during migration. Because öÇD varies geographically, the difference between animal tissue öÇD and post-migration water öÇD, after accounting for the biological fractionation of assimilation, can provide information regarding animal movement. In monarch butterflies, for example, wing chitin is metabolically inert after it is built, so it can reflect the isotopic composition of the environmental water at the time and location of wing growth. This then creates a record of butterfly origin and can be used to determine migration distance. This approach can also be used in bats and birds, using hair and feathers, respectively. Since rainwater becomes depleted as elevation increases, this method can also track altitudinal migration. However, this is technically hard to do, and the resolution seems to be too poor to track small altitudinal changes. H is most useful in tracking movement of species between areas with large continental water variation, since species movement can be complicated by the similarity of local water öÇD between places. For example, source water from Baja California may have the same öÇD as water from Maine. Further, a proportion of the HIC in the tissue can exchange with water and complicate the interpretation of measurements. To determine this percentage of isotopic exchange, which varies according to local humidity levels, standards of metabolically inert tissue from the species of interest can be constructed and equilibriated to local conditions. This allows measured öÇD from different regions to be compared against each other.Trophic interactions
Assimilation of diet into tissue has a tissue-specific fractionation known as the trophic discrimination factor. Diet sources can be tracked through a food web via deuterium isotope profiles, though this is complicated by deuterium having two potential sources ã water and food. Food more strongly impacts öÇD than does exchange with surrounding water, and that signal is seen across trophic levels. However, different organisms derive organic hydrogen in varying ratios of water to food: for example, in quail, 20-30% of organic hydrogen was from water and the remainder from food. The precise percentage of hydrogen from water, depended on tissue source and metabolic activity. In Chironomidae, chironomids, 31-47% of biomass hydrogen derived from water, and in microbes as much as 100% of fatty acid hydrogen can be derived from water depending on substrate. In caterpillars, diet öÇD from organic matter correlates linearly with tissue öÇD. The same relationship does not appear to hold consistently for diet öÇD from water, however ã water derived from either the caterpillar or its prey plant is more H-enriched than their organic material. Going up trophic levels from prey (plant) to predator (caterpillar) results in an isotopic enrichment. This same trend of enrichment is seen in many other animals - carnivores, omnivores, and herbivores - and seems to follow N relative abundances. Carnivores at the same trophic level tend to exhibit the same level of H enrichment. Because, as mentioned earlier, the amount of organic hydrogen produced from water varies between species, a model of trophic level related to absolute fractionation is difficult to make if the participating species are not known. Consistency in measuring the same tissues is also important, as different tissues fractionate deuterium differently. In aquatic systems, tracking trophic interactions is valuable for not only understanding the ecology of the system, but also for determining the degree of terrestrial input. The patterns of deuterium enrichment consistent within trophic levels is a useful tool for assessing the nature of these interactions in the environment.Microbial metabolism
Biological deuterium fractionation through metabolism is very organism and pathway dependent, resulting in a wide variability in fractionations. Despite this, some trends still hold. Hydrogen isotopes tend to fractionate very strongly in autotrophs relative to heterotrophs during lipid biosynthesis - chemoautotrophs produce extremely depleted lipids, with the fractionation ranging from roughly ã200 to ã400ã¯. This has been observed both in laboratory-grown cultures fed a known quantity of deuterated water and in the environment. Proteins, however, do not follow as significant a trend, with both heterotrophs and autotrophs capable of generating large and variable fractionations. In part, kinetic fractionation of the lighter isotope during formation of reducing equivalents Nicotinamide adenine dinucleotide, NADH and Nicotinamide adenine dinucleotide phosphate, NADPH result in lipids and proteins that are isotopically lighter. Salinity appears to play a role in the degree of deuterium fractionation as well; more saline waters affect growth rate, the rate of hydrogen exchange, and evaporation rate. All of these factors influence lipid öÇD upon hydrogen being incorporated into biomass. In coccolithophores ''Emiliania huxleyi'' and ''Gephyrocapsa oceanica'', alkenone öÇD has been found to correlate strongly to organism growth rate divided by salinity. The relationship between deuterium fractionation and salinity could potentially be used in #Alkenones as a paleosalinity proxy, paleoenvironment reconstruction with preserved lipids in the rock record to determine, for example, ocean salinity at the time of organismal growth. However, the degree of fractionation is not necessarily consistent between organisms, complicating the determination of paleosalinity with this method. There also appears to be a negative correlation between growth rate and fractionation in these coccolithophores. Further experiments on unicellular algae ''Eudorina unicocca'' and ''Volvox aureus'' show no effect of growth rate (controlled by nitrogen limitation) on fatty acid öÇD. However, sterols become more D-depleted as growth rate increases, in agreement with alkenone isotopic composition in coccolithophores. Overall, although there are some strong trends with lipid öÇD, the specific fractionations are compound-specific. As a result, any attempt to create a salinometer through öÇD measurements will necessarily be specific to a single compound type.Environmental chemistry
An important goal of environmental chemistry is tracing the source and degradation of pollutants. Various methods have been used for fingerprinting pools of environmental pollutants such as the bulk chemical composition of a spill, isotope ratios of the bulk chemical mixture, or isotope ratios of individual constituent compounds. Stable isotopes of carbon and hydrogen can be used as complementary fingerprinting techniques for natural gas. The DHR of hydrocarbons from the Deepwater Horizon oil spill was used to verify that they were likely from the Macondo well. HICs have also been used as a measure of the relative amount of biodegradation that has occurred in oil reservoirs in China, and studies on pure cultures of n-alkane degrading organisms have shown a chain-length dependence on the amount of hydrogen isotope fractionation during degradation. Additional studies have also shown hydrogen isotope effects in the degradation of methyl tert-butyl ether and toluene that have been suggested to be useful in the evaluation of the level of degradation of these polluting compounds in the environment. In both cases the residual unreacted compounds became H-enriched to a few tens of ã¯, with variations exhibited between different organisms and degree of reaction completeness. These observations of heavy residual compounds have been applied to field observations of biodegradation reactions such as removal of benzene and ethylbenzene, which imparted a D/H fractionation of 27 and 50 ã¯, respectively. Also, analysis of o-xylene in a polluted site showed high residual DHRs after biodegradation, consistent with activation of C-H bonds being a rate limiting step in this processSource attribution and forensics
Stable isotope ratios have found uses in various instances where the authenticity or origin of a chemical compound is called into question. Such situations include assessing the authenticity of food, wine and natural flavors; drug screening in sports (see doping in sport); pharmaceuticals; illicit drugs; and even helping identify human remains. In these cases it is often not enough to detect or quantify a certain compound, since the question is the origin of the compound. The strength of hydrogen isotope analysis in answering these questions is that the DHR of a natural product is often related to the natural water DHRs in the area where the product was formed (see: #Hydrologic cycle, Hydrologic cycle). Since DHRs vary significantly between different areas, this can be a powerful tool in locating the original source of many different substance.Food and flavor authentication
Foods, flavorings and scents are often sold with the guarantee that chemical additives come from natural sources. This claim becomes hard to test when the chemical compound has a known structure and is readily synthesized in a lab. Authentication of claims about the origins of these chemicals has made good use of various stable isotopes, including those of hydrogen. Combined carbon and hydrogen isotope analysis has been used to test the authenticity of (E)-methyl cinnamate, ö°-decalactone and öÇ-decalactone. Hydrogen and nitrogen isotope ratios have been used for the authentication of alkylpyrazines used as "natural" coffee flavorings.Doping
The isotope ratio of carbon in athletes' steroids has been used to determine whether these steroids came from the athlete's body or an outside source. This test has been used in a number of high-profile anti-doping cases and has various benefits over simply characterizing the concentration of various compounds. Attempts are being made to create similar tests based on stable hydrogen isotopes which could be used to complement the existing testing methods. One concern with this method was that the natural steroids produced by the human body may vary significantly based on the H content of drinking water, leading to false detection of doping based on HIC differences. This concern has been addressed in a recent study which concluded that the effect of DHR of drinking water did not pose an insurmountable source of error for this anti-doping testing strategy.Pharmaceutical copies
The pharmaceutical industry has revenues of hundreds of Pharmaceutical industry#Global sales, billions of dollars a year globally. With such a large industry Counterfeit medications, counterfeiting and copyright infringement are serious issues, and hydrogen isotope fingerprinting has become a useful tool in verifying the authenticity of various drugs. As described in the preceding sections, the utility of DHRs is highest when combined with measurements of other isotope ratios. In an early study on the stable isotope compositions of tropicamide, hydrocortisone, quinine and tryptophan; carbon, nitrogen, oxygen and hydrogen stable isotopes were analyzed by EA-IRMS; clear distinctions were able to be made between manufacturers and even batches of the drugs based on their isotope signatures. In this study it was determined that the hydrogen and oxygen isotope ratios were the two best fingerprints for distinguishing between different drug sources. A follow-up study analyzing naproxen from various lots and manufacturers also showed similar ability to distinguish between sources of the drugs. The use of these isotope signatures could not only be used to distinguish between different manufacturers, but also between different synthetic pathways for the same compound. These studies relied on the natural variations that occurred in the synthesis of these drugs, but other studies have used starting ingredients that are intentionally labeled D and C, and showed that these labels could be traced into the final pharmaceutical product. DHRs can also be determined for specific sites in a drug by H NMR, and has been used to distinguish between different synthetic methods for ibuprofen and naproxen in one study, and prozac and fluoxetine in another. These studies show that bulk DHR information for EA-IRMS, and site-specific DHRs from H NMR have great utility for pharmaceutical drug authenticity testing.Illicit drugs
The sources and production mechanisms of illegal drugs has been another area that has seen successful application of hydrogen isotope characterization. Usually, as with other applications of stable isotope techniques, results are best when data for multiple stable isotopes are compared with one another. öÇH, öÇC and öÇN have been used together to analyze tablets of 3,4-Methylenedioxyamphetamine, MDA and MDMA and has successfully identified differences which could be used to differentiate between different sources or production mechanisms. The same combination of stable isotopes with the addition of öÇO was applied to heroin and associated packaging and could successfully distinguish between different samples. Analysis using deuterium NMR was also able to shed light on the origin and processing of both cocaine and heroin. In the case of heroin this site-specific natural isotopic fraction measured by deuterium NMR (SNIF-NMR) method could be used for determining the geographic origin of the molecule by analyzing so-called natural sites (which were present in the morphine from which heroin is made), as well as gaining information on the synthesis process by analyzing the artificial sites (added during drug processing).Provenance of human remains
The geographic variation in DHR in human drinking water is recorded in hair. Studies have shown a very strong relation between an individual's hair and drinking water DHRs. Since tap water DHR depends strongly on geography, a person's hair DHR can be used to determine regions where they most likely lived during hair growth. This idea has been used in criminal investigations to try to constrain the movements of a person prior to their death, in much the same way DHRs have been used to track #Migration patterns, animal migration. By analyzing sections of hair of varying ages, one can determine in what D/H regions a person was living at a specific time before their death.See also
* Abundance of chemical elements * Hydrogen isotope * Natural abundance * Stable isotopesReferences
{{reflist, 25em