(trimethylsilyl)methyl on:
[Wikipedia]
[Google]
[Amazon]
(Trimethylsilyl)methyllithium is classified both as an
 (Trimethylsilyl)methyllithium is widely used in organotransition metal chemistry to affix (trimethylsilyl)methyl ligands. Such complexes are usually produced by
(Trimethylsilyl)methyllithium is widely used in organotransition metal chemistry to affix (trimethylsilyl)methyl ligands. Such complexes are usually produced by
organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
and an organosilicon compound
Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, f ...
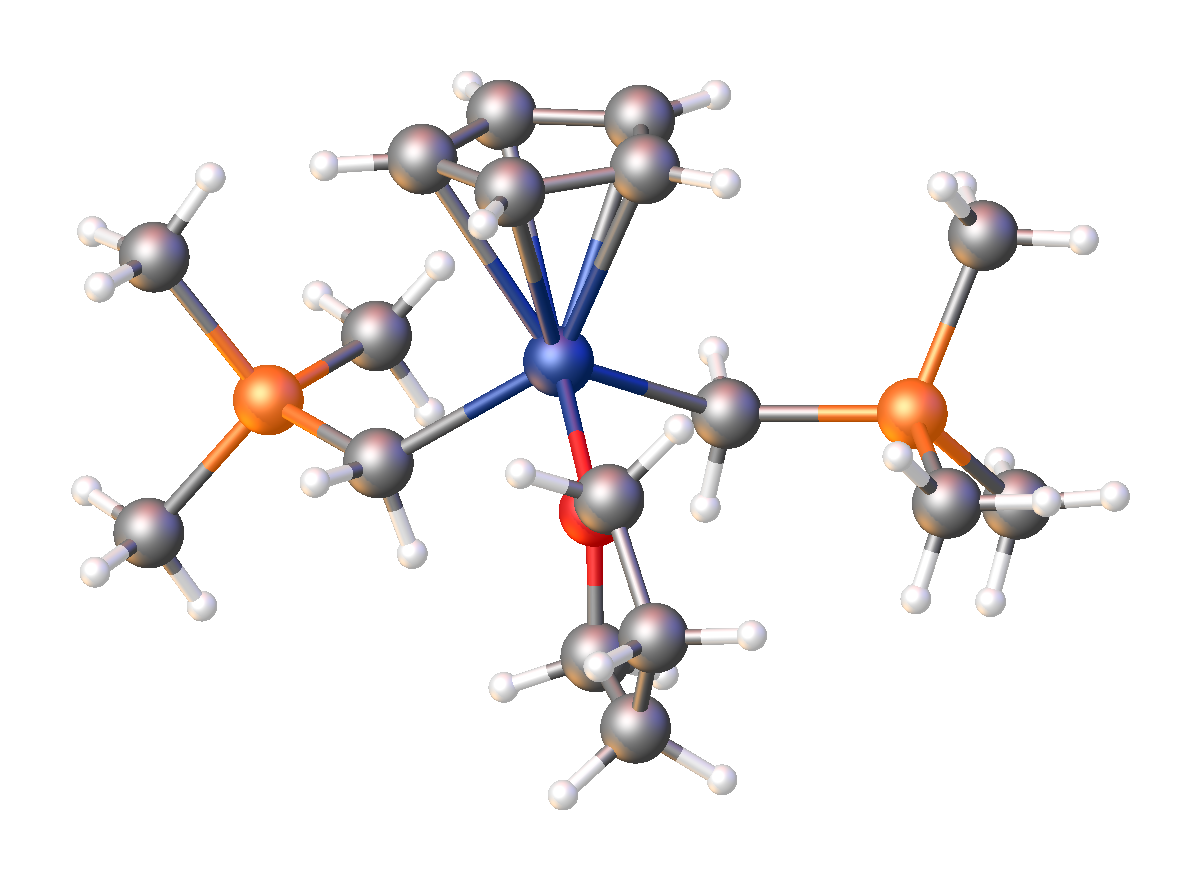
. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2TMS. It crystallizes as the hexagonal prism
In geometry, the hexagonal prism is a Prism (geometry), prism with hexagonal base. Prisms are polyhedrons; this polyhedron has 8 face (geometry), faces, 18 Edge (geometry), edges, and 12 vertex (geometry), vertices..
As a semiregular polyhedro ...
atic hexamer iCH2TMSsub>6, akin to some polymorphs of methyllithium
Methyllithium is the simplest organolithium reagent, with the empirical formula LiCH3. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used i ...
. Many adducts have been characterized including the diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
complexed cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substanc ...
i4(μ3-CH2TMS)4(Et2O)2and TMEDA
Tetramethylethylenediamine (TMEDA or TEMED) is a chemical compound with the formula (CH3)2NCH2CH2N(CH3)2. This species is derived from ethylenediamine by replacement of the four amine hydrogens with four methyl groups. It is a colorless liquid, ...
)2].
Preparation
(Trimethylsilyl)methyllithium, which is commercially available as a THF solution, is usually prepared by treatment of (Trimethylsilyl)methyl chloride, (trimethylsilyl)methyl chloride withbutyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
:
:(CH3)3SiCH2Cl + BuLi → (CH3)3SiCH2Li + BuCl
(Trimethylsilyl)methylmagnesium chloride is often functionally equivalent to (trimethylsilyl)methyllithium. It is prepared by the Grignard reaction
The Grignard reaction () is an organometallic chemical reaction in which, according to the classical definition, carbon alkyl, allyl, vinyl, or aryl magnesium halides (Grignard reagent) are added to the carbonyl groups of either an aldehyde or ...
of (trimethylsilyl)methyl chloride.
Use in methylenations
In one example of the Peterson olefination, (trimethylsilyl)methyllithium reacts withaldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
s and ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s to give the terminal alkene
In organic chemistry, terminal alkenes (alpha-olefins, α-olefins, or 1-alkenes) are a family of organic compounds which are alkenes (also known as olefins) with a chemical formula , distinguished by having a double bond at the primary, alpha ( ...
(R1 = Me, R2 & R3 = H):
:
Metal derivatives
 (Trimethylsilyl)methyllithium is widely used in organotransition metal chemistry to affix (trimethylsilyl)methyl ligands. Such complexes are usually produced by
(Trimethylsilyl)methyllithium is widely used in organotransition metal chemistry to affix (trimethylsilyl)methyl ligands. Such complexes are usually produced by salt metathesis
A salt metathesis reaction (also called a double displacement reaction, double replacement reaction, or double decomposition) is a type of chemical reaction in which two ionic compounds in aqueous solution exchange their component ions to form two ...
involving metal chloride
In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
Bonding
Halides are X-type ligands in coordination ch ...
s. These compounds are often highly soluble in nonpolar organic solvents, enjoying stability due to their steric bulk
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
and resistance to beta-hydride elimination. In these regards, (trimethylsilyl)methyl is akin to neopentyl
Pentyl is a five-carbon alkyl group or substituent with chemical formula . It is the substituent form of the alkane pentane.
In older literature, the common non-systematic name amyl was often used for the pentyl group. Conversely, the name penty ...
.
Bis(trimethylsilyl)methylmagnesium is used as an alternative to (trimethylsilyl)methyllithium.
Related compounds
* bis(trimethylsilyl)methyllithium * tris(trimethylsilyl)methyllithiumReferences
{{DEFAULTSORT:Trimethylsilyl methyllithium Organolithium compounds Trimethylsilyl compounds