|
Zero Field Splitting
Zero-field splitting (ZFS) describes various interactions of the energy levels of a molecule or ion resulting from the presence of more than one unpaired electron. In quantum mechanics, an energy level is called degenerate if it corresponds to two or more different measurable states of a quantum system. In the presence of a magnetic field, the Zeeman effect is well known to split degenerate states. In quantum mechanics terminology, the degeneracy is said to be "lifted" by the presence of the magnetic field. In the presence of more than one unpaired electron, the electrons mutually interact to give rise to two or more energy states. Zero-field splitting refers to this lifting of degeneracy even in the absence of a magnetic field. ZFS is responsible for many effects related to the magnetic properties of materials, as manifested in their electron spin resonance spectra and magnetism. The classic case for ZFS is the spin triplet, i.e., the ''S'' = 1 spin system. In the presen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Dipole–dipole Interaction
Magnetic dipole–dipole interaction, also called dipolar coupling, refers to the direct interaction between two magnetic dipoles. Roughly speaking, the magnetic dipole#External magnetic field produced by a magnetic dipole moment, magnetic field of a dipole goes as the inverse cube of the distance, and the Force between magnets#Magnetic force due to non-uniform magnetic field, force of its magnetic field on another dipole goes as the first derivative of the magnetic field. It follows that the dipole-dipole interaction goes as the inverse fourth power of the distance. Suppose and are two magnetic dipole moments that are far enough apart that they can be treated as point dipoles in calculating their interaction energy. The potential energy of the interaction is then given by: :: H = -\frac\left[ 3(\mathbf m_1\cdot\hat\mathbf r)(\mathbf m_2\cdot\hat\mathbf r) - \mathbf m_1\cdot\mathbf m_2\right]-\mu_0 \frac \mathbf m_1\cdot\mathbf m_2 \delta(\mathbf r), :: where is the magneti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
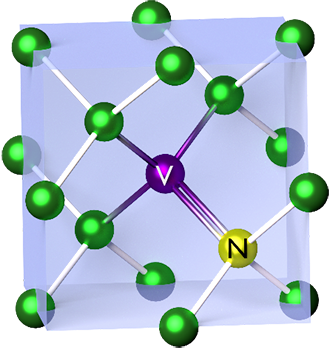
N-V Center
The nitrogen-vacancy center (N-V center or NV center) is one of numerous photoluminescent point defects in diamond. Its most explored and useful properties include its spin-dependent photoluminescence (which enables measurement of the electronic spin state using optically detected magnetic resonance), and its relatively long spin coherence at room temperature, lasting up to milliseconds. The NV center energy levels are modified by magnetic fields, electric fields, temperature, and strain, which allow it to serve as a sensor of a variety of physical phenomena. Its atomic size and spin properties can form the basis for useful quantum sensors. NV centers enable nanoscale measurements of magnetic and electric fields, temperature, and mechanical strain with improved precision. External perturbation sensitivity makes NV centers ideal for applications in biomedicine—such as single-molecule imaging and cellular process modeling. NV centers can also be initialized as qubits and enable th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the Chemical stability, chemically stable form of carbon at Standard temperature and pressure, room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest Scratch hardness, hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of lattice defect, defects or impurities (about one per million of lattice atoms) can color ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorescence
Phosphorescence is a type of photoluminescence related to fluorescence. When exposed to light (radiation) of a shorter wavelength, a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. Unlike fluorescence, a phosphorescent material does not immediately reemit the radiation it absorbs. Instead, a phosphorescent material absorbs some of the radiation energy and reemits it for a much longer time after the radiation source is removed. In a general sense, there is no distinct boundary between the emission times of fluorescence and phosphorescence (i.e.: if a substance glows under a black light it is generally considered fluorescent, and if it glows in the dark it is often simply called phosphorescent). In a modern, scientific sense, the phenomena can usually be classified by the three different mechanisms that produce the light, and the typical timescales during which those mechanisms emit light. Whereas fluorescent materials stop emitti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SQUID
A squid (: squid) is a mollusc with an elongated soft body, large eyes, eight cephalopod limb, arms, and two tentacles in the orders Myopsida, Oegopsida, and Bathyteuthida (though many other molluscs within the broader Neocoleoidea are also called ''squid'' despite not strictly fitting these criteria). Like all other cephalopods, squid have a distinct head, Symmetry (biology)#Bilateral symmetry, bilateral symmetry, and a mantle (mollusc), mantle. They are mainly soft-bodied, like octopuses, but have a small internal skeleton in the form of a rod-like gladius (cephalopod), gladius or pen, made of chitin. Squid diverged from other cephalopods during the Jurassic and occupy a similar Ecological niche, role to teleost fish as open-water predators of similar size and behaviour. They play an important role in the open-water food web. The two long tentacles are used to grab prey and the eight arms to hold and control it. The beak then cuts the food into suitable size chunks for swal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauli Matrices
In mathematical physics and mathematics, the Pauli matrices are a set of three complex matrices that are traceless, Hermitian, involutory and unitary. Usually indicated by the Greek letter sigma (), they are occasionally denoted by tau () when used in connection with isospin symmetries. \begin \sigma_1 = \sigma_x &= \begin 0&1\\ 1&0 \end, \\ \sigma_2 = \sigma_y &= \begin 0& -i \\ i&0 \end, \\ \sigma_3 = \sigma_z &= \begin 1&0\\ 0&-1 \end. \\ \end These matrices are named after the physicist Wolfgang Pauli. In quantum mechanics, they occur in the Pauli equation, which takes into account the interaction of the spin of a particle with an external electromagnetic field. They also represent the interaction states of two polarization filters for horizontal/vertical polarization, 45 degree polarization (right/left), and circular polarization (right/left). Each Pauli matrix is Hermitian, and together w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unpaired Electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain two electrons ( electron pair) with opposite spins. As the formation of electron pairs is often energetically favourable, either in the form of a chemical bond or as a lone pair, unpaired electrons are relatively uncommon in chemistry, because an entity that carries an unpaired electron is usually rather reactive. In organic chemistry they typically only occur briefly during a reaction on an entity called a radical; however, they play an important role in explaining reaction pathways. Radicals are uncommon in s- and p-block chemistry, since the unpaired electron occupies a valence p orbital or an sp, sp2 or sp3 hybrid orbital. These orbitals are strongly directional and therefore overlap to form strong covalent bonds, favouring dime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamiltonian (quantum Mechanics)
In quantum mechanics, the Hamiltonian of a system is an operator corresponding to the total energy of that system, including both kinetic energy and potential energy. Its spectrum, the system's ''energy spectrum'' or its set of ''energy eigenvalues'', is the set of possible outcomes obtainable from a measurement of the system's total energy. Due to its close relation to the energy spectrum and time-evolution of a system, it is of fundamental importance in most formulations of quantum theory. The Hamiltonian is named after William Rowan Hamilton, who developed a revolutionary reformulation of Newtonian mechanics, known as Hamiltonian mechanics, which was historically important to the development of quantum physics. Similar to vector notation, it is typically denoted by \hat, where the hat indicates that it is an operator. It can also be written as H or \check. Introduction The Hamiltonian of a system represents the total energy of the system; that is, the sum of the kine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeeman Splitting
The Zeeman effect () is the splitting of a spectral line into several components in the presence of a static magnetic field. It is caused by the interaction of the magnetic field with the magnetic moment of the atomic electron associated with its orbital motion and spin; this interaction shifts some orbital energies more than others, resulting in the split spectrum. The effect is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel Prize in Physics for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also, similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden (in the dipole approximation), as governed by the selection rules. Since the distance between the Zeeman sub-levels is a function of magnetic field strength, this effect can be used to measure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |