|
Xanthine Oxidoreductase
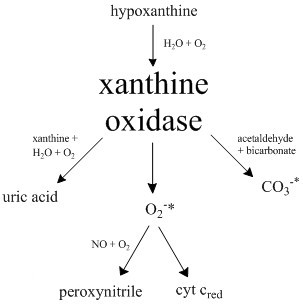
Xanthine oxidase (XO or XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. "XDH xanthine dehydrogenase" Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 xanthine + H2O2 * xanthine + H2O + O2 uric acid + H2O2 * Xanthine oxidase can a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine Dehydrogenase
Xanthine dehydrogenase, also known as XDH, is a protein that, in humans, is encoded by the ''XDH'' gene. Function Xanthine dehydrogenase belongs to the group of molybdenum-containing hydroxylases involved in the oxidative metabolism of purines. The enzyme is a homodimer. Xanthine dehydrogenase can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Xanthine dehydrogenase catalyzes the following chemical reaction: xanthine + NAD+ + H2O \rightleftharpoons urate + NADH + H+ The three substrates of this enzyme are xanthine, NAD+, and H2O, whereas its three products are urate, NADH, and H+. This enzyme participates in purine metabolism. Nomenclature This enzyme belongs to the family of oxidoreductases, to be specific, those acting on CH or CH2 groups with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is xanthine:NAD+ oxidoreductase. Other names in common use include NAD+-xanthine dehydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Anhydrase
The carbonic anhydrases (or carbonate dehydratases) () form a family of enzymes that catalyst, catalyze the interconversion between carbon dioxide and water and the Dissociation (chemistry), dissociated ions of carbonic acid (i.e. bicarbonate and hydrogen ions). The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloprotein, metalloenzymes. The enzyme maintains Acid–base homeostasis, acid-base balance and helps transport carbon dioxide. Carbonic anhydrase helps maintain acid–base homeostasis, regulate pH, and fluid balance. Depending on its location, the role of the enzyme changes slightly. For example, carbonic anhydrase produces acid in the stomach lining. In the kidney, the control of bicarbonate ions influences the water content of the cell. The control of bicarbonate ions also influences the water content in the eyes. Inhibitors of carbonic anhydrase are used to treat glaucoma, the excessive build-up of water in the eyes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serum (blood)
Serum () is the fluid and solvent component of blood which does not play a role in clotting. It may be defined as blood plasma without the clotting factors, or as blood with all cells and clotting factors removed. Serum contains all proteins except clotting factors (involved in blood clotting), including all electrolytes, antibodies, antigens, hormones; and any exogenous substances (e.g., drugs, microorganisms). Serum also does not contain all the formed elements of blood, which include blood cells, white blood cells ( leukocytes, lymphocytes), red blood cells ( erythrocytes), and platelets. The study of serum is serology. Serum is used in numerous diagnostic tests as well as blood typing. Measuring the concentration of various molecules can be useful for many applications, such as determining the therapeutic index of a drug candidate in a clinical trial. To obtain serum, a blood sample is allowed to clot ( coagulation). The sample is then centrifuged to remove th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (). Others are: * Atomic oxygen (), a free radical. * Singlet oxygen (), one of two metastable states of molecular oxygen. * Tetraoxygen (), another metastable form. * Solid oxygen, existing in six variously colored phases, of which one is octaoxygen (, red oxygen) and another one metallic (ζ-oxygen). Atomic oxygen Atomic oxygen, denoted O or O1, is very reactive, as the individual atoms of oxygen tend to quickly bond with nearby molecules. Its lowest-energy electronic state is a spin triplet, designated by the term symbol 3P. On Earth's surface, it exists naturally for a very short time. In outer space, the presence of ample ultraviolet radiation results in a low Earth orbit atmosphere in which 96% of the oxygen occurs in atomic form. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substrate (biochemistry)
In chemistry, the term substrate is highly context-dependent. Broadly speaking, it can refer either to a chemical species being observed in a chemical reaction, or to a surface on which other chemical reactions or microscopy are performed. In the former sense, a reagent is added to the ''substrate'' to generate a product through a chemical reaction. The term is used in a similar sense in synthetic and organic chemistry, where the substrate is the chemical of interest that is being modified. In biochemistry, an enzyme substrate is the material upon which an enzyme acts. When referring to Le Chatelier's principle, the substrate is the reagent whose concentration is changed. ;Spontaneous reaction : :*Where S is substrate and P is product. ;Catalysed reaction : :*Where S is substrate, P is product and C is catalyst. In the latter sense, it may refer to a surface on which other chemical reactions are performed or play a supporting role in a variety of spectroscopic and mic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. Hydroxide ion The hydroxide ion is naturally produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic organism, anaerobic bacterium ''Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight-Electron excitation, excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdopterin
Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin itself contains no molybdenum; rather, this is the name of the ligand (a ''pterin'') that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor. Molybdopterin is required for all forms of life. Molybdopterin consists of a pyranopterin, a complex heterocycle featuring a pyran fused to a pterin ring. In addition, the pyran ring features two thiolates, which serve as ligands in molybdo- and tungstoenzymes. In some cases, the alkyl phosphate group is replaced by an alkyl diphosphate nucleotide. Enzymes that contain the molybdopterin cofactor include xanthine oxidase, DMSO reductase, sulfite oxidase, and nitrate reductase. The only molyb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a Native metal, free metal on Earth; in its minerals, it is found only in oxidation state, oxidized states. The free element, a silvery metal with a grey cast, has the List of elements by melting point, sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |