|
Xanthates
150px, Sodium salt of ethyl xanthate Xanthate usually refers to a salt with the formula (R = alkyl; M+ = Na+, K+), thus they are the metal-thioate/''O''-esters of dithiocarbonate. The name ''xanthates'' is derived from Ancient Greek ''xanthos'', meaning “yellowish, golden”, and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and (in mining) for extraction of certain ores. They are also versatile intermediates in organic synthesis. ''Xanthate'' can also refer to the ''O'',''S''-ester of xanthic acid. These esters have the structure ROC(=S)SR′. Formation and structure Xanthate salts are produced by the treatment of an alcohol, alkali, and carbon disulfide. The process is called xanthation. In chemical terminology, the alkali reacts with the alcohol to produce an alkoxide, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Ethyl Xanthate
Sodium ethyl xanthate (SEX) is an organosulfur compound with the chemical formula . It is a pale yellow powder, which is usually obtained as the dihydrate. Sodium ethyl xanthate is used in the mining industry as a flotation agent. A closely related potassium ethyl xanthate (KEX) is obtained as the anhydrous salt. Production Akin to the preparation of most xanthates, sodium ethyl xanthate can be prepared by treating sodium ethoxide with carbon disulfide: Properties and reactions Sodium ethyl xanthate is a pale yellow powder. Its aqueous solutions are stable at high pH if not heated. It rapidly hydrolyses at pH <9 at 25 °C. It is the conjugate base of the unknown strong acid with p''K''a of 1.6 and p''K''b estimated as 12.4 for the . Sodium ethyl xanthate easily adsorbs on the surface of many sulfide minerals, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chugaev Elimination
The Chugaev elimination is a chemical reaction that involves the elimination of water from alcohols to produce alkenes. The intermediate is a xanthate. It is named for its discoverer, the Russian chemist Lev Aleksandrovich Chugaev (1873-1922), who first reported the reaction sequence in 1899. In the first step, a xanthate salt is formed out of the alkoxide and carbon disulfide (CS2). With the addition of iodomethane, the alkoxide is transformed into a methyl xanthate. At about 200 °C, the alkene is formed by an intramolecular elimination. In a 6-membered cyclic transition state the hydrogen atom is removed from the carbon atom β to the xanthate oxygen in a ''syn''-elimination. The side product decomposes to carbonyl sulfide (OCS) and methanethiol. The Chugaev elimination is similar in mechanism to other thermal elimination reactions such as the Cope elimination and ester pyrolysis. Xanthates typically undergo elimination from 120 to 200 °C, while esters typic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Amyl Xanthate
Potassium amyl xanthate (/pəˈtæsiəm ˌæmɪl ˈzænθeɪt/) is an organosulfur compound with the chemical formula CH3(CH2)4OCS2K. It is a pale yellow powder with a pungent odor that is soluble in water. It is widely used in the mining industry for the separation of ores using the flotation process. Production and properties As typical for xanthates, potassium amyl xanthate is prepared by reacting ''n''-amyl alcohol with carbon disulfide and potassium hydroxide Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash. Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which expl .... : CH3(CH2)4OH + CS2 + KOH → CH3(CH2)4OCS2K + H2O Potassium amyl xanthate is a pale yellow powder. Its solutions are relatively stable between pH 8 and 13 with a maximum of stability at pH 10. Related compounds *Sodium amyl xanthate is used in the separation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Ethyl Xanthate
Potassium ethyl xanthate (KEX) is an organosulfur compound with the chemical formula CH3CH2OCS2K. It is a pale yellow powder that is used in the mining industry for the separation of ores. Unlike the related sodium ethyl xanthate, the potassium salt exists as an anhydrous salt. Production and properties Xanthate salts are prepared by the action of alkoxides on carbon disulfide. The alkoxide is often generated in situ from potassium hydroxide: :EtOH + CS2 + KOH → EtOCS2K + H2O Potassium ethyl xanthate is a pale yellow powder that is stable at high pH but rapidly hydrolyses below pH <9: : Oxidation gives diethyl dixanthogen disulfide: : KEX is a source of ethylxanthate es. For example have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
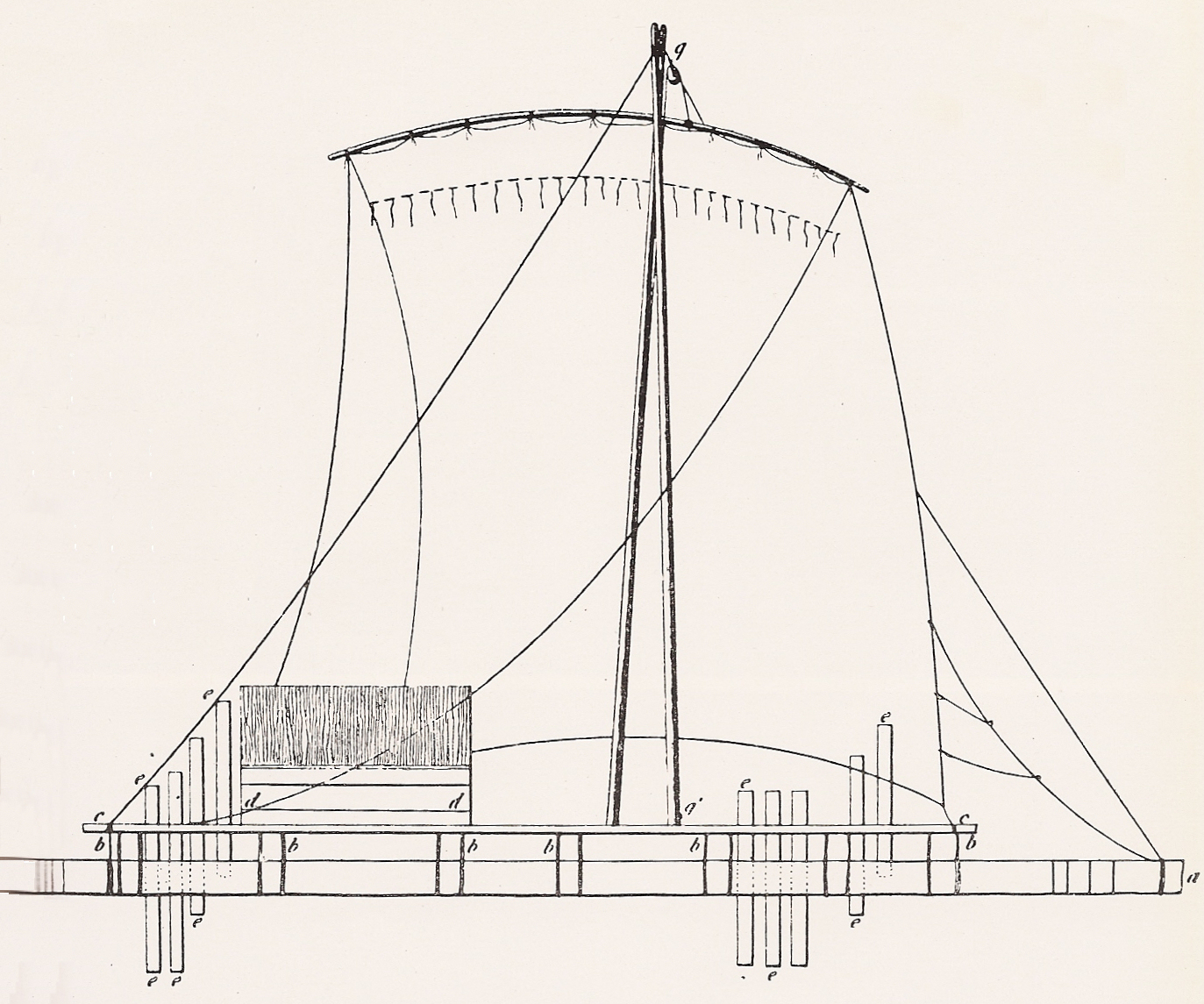
RAFT (chemistry)
A raft is any flat structure for support or transportation over water. It is usually of basic design, characterized by the absence of a hull. Rafts are usually kept afloat by using any combination of buoyant materials such as wood, sealed barrels, or inflated air chambers (such as pontoons), and are typically not propelled by an engine. Rafts are an ancient mode of transport; naturally-occurring rafts such as entwined vegetation and pieces of wood have been used to traverse water since the dawn of humanity. Human-made rafts Traditional or primitive rafts were constructed of wood or reeds. Modern rafts may also use pontoons, drums, or extruded polystyrene blocks. Inflatable rafts up to the 20th century used flotation chambers made of goat- or buffalo-skins, but most now use durable, multi-layered rubberized fabrics. Depending on its use and size, it may have a superstructure, masts, or rudders. Timber rafting is used by the logging industry for the transportation of logs, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Living Polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar (i.e. they have a very low polydispersity index). Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups. Living polymerization is desirable because it offers precision and control in macromolecular synthesis. This is important since many of the novel/useful properties of polymers result ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
M(S2COEt)3
M, or m, is the thirteenth letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''em'' (pronounced ), plural ''ems''. History The letter M is derived from the Phoenician Mem, via the Greek Mu (Μ, μ). Semitic Mem is most likely derived from a " Proto-Sinaitic" (Bronze Age) adoption of the "water" ideogram in Egyptian writing. The Egyptian sign had the acrophonic value , from the Egyptian word for "water", ''nt''; the adoption as the Semitic letter for was presumably also on acrophonic grounds, from the Semitic word for "water", '' *mā(y)-''. Use in writing systems The letter represents the bilabial nasal consonant sound in the orthography of Latin as well as in that of many modern languages, and also in the International Phonetic Alphabet. In English, the Oxford English Dictionary (first edition) says that is sometimes a vowel, in words like ''spasm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dixanthogen Disulfide
: Structure of diethyl dixanthogen disulfide. Dixanthogen disulfides are a class of organosulfur compounds with the formula . Usually yellow solids, they are the product of the oxidation of xanthate salts. A common derivative is diethyl dixanthogen disulfide. Diisopropyl dixanthogen disulfide is commercially available. They are structurally related to thiuram disulfides. Uses and reactions Diethyl dixanthogen disulfide is a component for froth flotations used, inter alia, for the separation of sulfide minerals like pyrrhotite. Diisopropyl dixanthogen disulfide is a reagent in the synthesis of sulfur heterocycles. Dialkoxy dixanthogen disulfides undergo desulfurization by cyanide to give bis(alkoxythiocarbonyl)sulfides: : Dixanthogens are also ectoparasiticide An ectoparasiticide is an antiparasitic drug used in the treatment of ectoparasitic infestations. These drugs are used to kill the parasites that live on the body surface. Permethrin, sulfur, lindane, dicophane, benzyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barton–McCombie Deoxygenation
The Barton–McCombie deoxygenation is an organic reaction in which a hydroxy functional group in an organic compound is replaced by a hydrogen to give an alkyl group. It is named after British chemists Sir Derek Harold Richard Barton and Stuart W. McCombie. This deoxygenation reaction is a radical substitution. In the related Barton decarboxylation the reactant is a carboxylic acid. Mechanism The reaction mechanism consists of a catalytic radical initiation step and a propagation step. The alcohol (1) is first converted into a reactive carbonothioyl intermediate such as a thionoester or xanthate 2. Heating of AIBN results in its homolytic cleavage, generating two 2-cyanoprop-2-yl radicals 9 which each abstract a proton from tributylstannane 3 to generate tributylstannyl radicals 4 and inactive 10. The tributyltin radical abstracts the xanthate group from 2 by attack of 4 at the sulfur atom with concurrent homolytic cleavage of the C-S π bond. This leaves a carbon cen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new cova ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)