|
Von Braun Reaction
The von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide. An example is the reaction of ''N'',''N''-dimethyl-1-naphthylamine: These days, most chemist have replaced cyanogen bromide reagent with chloroethyl chloroformate reagent instead. It appears as though Olofson et al. was the first chemist to have reported this. Reaction mechanism The reaction mechanism consists of two nucleophilic substitutions: the amine is the first nucleophile displacing the bromine atom which then acts as the second nucleophile. In following the mechanism is described using trimethylamine as example: First, the trimethylamine reacts with the cyanogen bromide to form a quaternary ammonium salt, which in the next step reacts by splitting off bromomethane to give the dimethylcyanamide. This is a bimolecular nucleophilic substitution ( SN2). See also * von Braun amide degradation The von Braun amide degradation is the chemical reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Von Braun Amide Degradation
The von Braun amide degradation is the chemical reaction of a monosubstituted amide with phosphorus pentachloride or thionyl chloride to give a nitrile and an organohalide. It is named after Julius Jacob von Braun, who first reported the reaction. Reaction mechanism The secondary amide 1 reacts via its enolized form with phosphorus pentachloride to form the oxonium ion 2. This produces a chloride ion which deprotonates the oxonium ion to form and imine 3 and hydrogen chloride. These then react with one another to form an amine, with loss of the phosphorus chloride residue. The β-chloroimine 4 is unstable and undergoes internal elimination to a form a nitrilium cation 5 which is cleaved by attack by chloride to form a nitrile 6a and a haloalkane 6b. See also *von Braun reaction *:de:Julius von Braun (Chemiker), Julius von Braun *Rosenmund–von Braun reaction References {{Organic reactions Elimination reactions Degradation reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
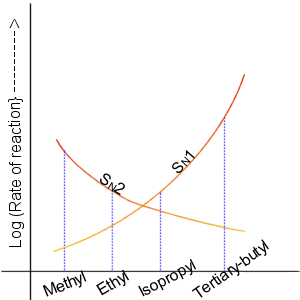
SN2 Reaction
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted (i.e. simultaneous) fashion. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bimolecular mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement of the leaving group, which is the rate-determining step, is separate from the nucleophilic attack in SN1. The SN2 reaction can be considered as an organic-chemistry analogue of the associative substitution from the field of inorganic chemistry. Reaction mech ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromomethane
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula C H3 Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It is a recognized ozone-depleting chemical. According to the IPCC Fifth Assessment Report, it has a global warming potential of 2. The compound was used extensively as a pesticide until being phased out by most countries in the early 2000s. From a chemistry perspective, it is one of the halomethanes. Occurrence and manufacture Marine organisms are estimated to produce 56,000 tonnes annually. It is also produced in small quantities by certain terrestrial plants, such as members of the family Brassicaceae. In 2009, an estimated 24,000 tonnes of methyl bromide were produced. Its production was curtailed by the Montreal Protocol, such that in 1983, production was nearly twice that of 2009 levels. It is manufactured by treating methanol with bromine in the presence of sulfur or hydrogen sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Von Braun Reaction
The von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide. An example is the reaction of ''N'',''N''-dimethyl-1-naphthylamine: These days, most chemist have replaced cyanogen bromide reagent with chloroethyl chloroformate reagent instead. It appears as though Olofson et al. was the first chemist to have reported this. Reaction mechanism The reaction mechanism consists of two nucleophilic substitutions: the amine is the first nucleophile displacing the bromine atom which then acts as the second nucleophile. In following the mechanism is described using trimethylamine as example: First, the trimethylamine reacts with the cyanogen bromide to form a quaternary ammonium salt, which in the next step reacts by splitting off bromomethane to give the dimethylcyanamide. This is a bimolecular nucleophilic substitution ( SN2). See also * von Braun amide degradation The von Braun amide degradation is the chemical reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a trimethylated derivative of ammonia. TMA is widely used in industry. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes on contact. At lower concentrations, it has a "fishy" odor, the odor associated with rotting fish. Physical and chemical properties TMA is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. It is also sold in pressurized gas cylinders. TMA protonates to give the trimethylammonium cation. Trimethylamine is a good nucleophile, and this reactivity underpins most of its applications. Trimethylamine is a Lewis base that forms adducts with a variety of Lewis acids. Production Industry and laboratory Trimethylamine is prepared by the reaction of ammonia and methanol employing a catalyst: :3 CH3OH + NH3 → (CH3)3N + 3 H2O This reaction coproduces the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Reactions
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions. The oldest organic reactions are combustion of organic fuels and saponification of fats to make soap. Modern organic chemistry starts with the Wöhler synthesis in 1828. In the history of the Nobel Prize in Chemistry awards have been given for the invention of specific organic reactions such as the Grignard reaction in 1912, the Diels–Alder reaction in 1950, the Wittig reaction in 1979 and olefin metathesis in 2005. Classifications Organic chemistry has a strong tradition of naming a specif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen Bromide
Cyanogen bromide is the inorganic compound with the chemical formula, formula BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a pseudohalogen. Synthesis, basic properties, and structure The carbon atom in cyanogen bromide is bonded to bromine by a single bond and to nitrogen by a triple bond (i.e. ). The compound is linear and polar, but it does not spontaneously ionize in water. It dissolves in both water and polar organic solvents. Cyanogen bromide can be prepared by oxidation of sodium cyanide with bromine, which proceeds in two steps via the intermediate cyanogen (): : : When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to cyanuric bromide (). This reaction is catalyzed by traces of bromine, metal salts, acids and bases. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroethyl Chloroformate (Dalapon)
{{MolFormIndex ...
The molecular formula C3H4Cl2O2 (molar mass: 142.97 g/mol, exact mass: 141.9588 u) may refer to: * 1-Chloroethyl chloroformate * 2-Chloroethyl chloroformate * 2,2-Dichloropropionic acid 2,2-Dichloropropionic acid is the organic compound with the formula CH3CCl2CO2H. It is a colorless liquid that freezes near room temperature. Occurrence and use Its sodium salt once was marketed under the name Dalapon as a selective herbicide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |