|
Vitamin K2
Vitamin K2 or menaquinone (MK) () is one of three types of vitamin K, the other two being vitamin K1 ( phylloquinone) and K3 (menadione). K2 is both a tissue and bacterial product (derived from vitamin K1 in both cases) and is usually found in animal products or fermented foods. The number ''n'' of isoprenyl units in their side chain differs and ranges from 4 to 13, hence vitamin K2 consists of various forms. It is indicated as a suffix (-n), e. g. MK-7 or MK-9. * The most common in the human diet is the short-chain, water-soluble menatetrenone (MK-4), which is commonly found in animal products. However, at least one published study concluded that "MK-4 present in food does not contribute to the vitamin K status as measured by serum vitamin K levels." The MK-4 in animal (including human) tissue is made from dietary plant vitamin K1. This process can be accomplished by animal tissues alone, as it proceeds in germ-free rodents. * Long-chain menaquinones (longer than MK-4) include ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers are the main component of natural rubber. History and etymology Charles Greville Williams, C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber. He correctly deduced the mass shares of carbon and hydrogen (but arrived at an incorrect formula C10H8 because the modern atomic weight of carbon was not adopted until the Karlsruhe Congress held later that year). He did not specify the reasons for the name, but it is hypothesized that it came from "propylene" with which isoprene shares some physical and chemical properties. The first one to observe recombination of isoprene into rubber-like substance was in 1879, and William A. Tilden identified its structure five years later. Natural occurrences Is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor IX
Factor IX (), also known as Christmas factor, is one of the serine proteases involved in coagulation; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia. Coagulation factor IX is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Physiology Factor IX is produced as a zymogen, an inactive precursor. It is processed to remove the signal peptide, Glycosylation, glycosylated and then cleaved by factor XIa (of the contact pathway) or factor VIIa (of the tissue factor pathway) to produce a two-chain form, where the chains are linked by a disulfide bridge. When activated into factor IXa, in the presence of Ca2+, membrane phospholipids, and a Factor VIII cofactor, it hydrolyses one arginine-isoleucine bond in factor X to form factor Xa. Factor IX is inhibited by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor VII
Coagulation factor VII (, formerly known as proconvertin) is a protein involved in coagulation and, in humans, is encoded by gene ''F7''. It is an enzyme of the serine protease class. Once bound to tissue factor released from damaged tissues, it is converted to factor VIIa (or ''blood-coagulation factor VIIa'', ''activated blood coagulation factor VII''), which in turn activates factor IX and factor X. Using genetic recombination a recombinant factor VIIa (eptacog alfa) (trade names include NovoSeven) has been approved by the FDA for the control of bleeding in hemophilia. It is sometimes used unlicensed in severe uncontrollable bleeding, although there have been safety concerns. A biosimilar form of recombinant activated factor VII (AryoSeven) is also available, but does not play any considerable role in the market. In April 2020, the US FDA approved a new rFVIIa product, eptacog beta (SEVENFACT), the first bypassing agent (BPA) approved in more than 2 decades. As an rFVIIa pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor II
Prothrombin (coagulation factor II) is encoded in the human by the F2-gene. It is proteolytically cleaved during the clotting process by the prothrombinase enzyme complex to form thrombin. Thrombin (Factor IIa) (, fibrose, thrombase, thrombofort, topical, thrombin-C, tropostasin, activated blood-coagulation factor II, E thrombin, beta-thrombin, gamma-thrombin) is a serine protease, that converts fibrinogen into strands of insoluble fibrin, as well as catalyzing many other coagulation-related reactions. History After the description of fibrinogen and fibrin, Alexander Schmidt hypothesised the existence of an enzyme that converts fibrinogen into fibrin in 1872. Prothrombin was discovered by Pekelharing in 1894. Physiology Synthesis Thrombin is produced by the enzymatic cleavage of two sites on prothrombin by activated Factor X (Xa). The activity of factor Xa is greatly enhanced by binding to activated Factor V (Va), termed the prothrombinase complex. Prothrombin is produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gla Domain
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions. The GLA domain binds calcium ions by chelating them between two carboxylic acid residues. These residues are part of a region that starts at the N-terminal extremity of the mature form of Gla proteins, and that ends with a conserved aromatic residue. This results in a conserved Gla-x(3)-Gla-x-Cys motif that is found in the middle of the domain, and which seems to be important for substrate recognition by the carboxylase. The 3D structures of several Gla domains have been solved. Calcium ions induce conformational changes in the Gla domain and are necessary for the Gla domain to fold properly. A common structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
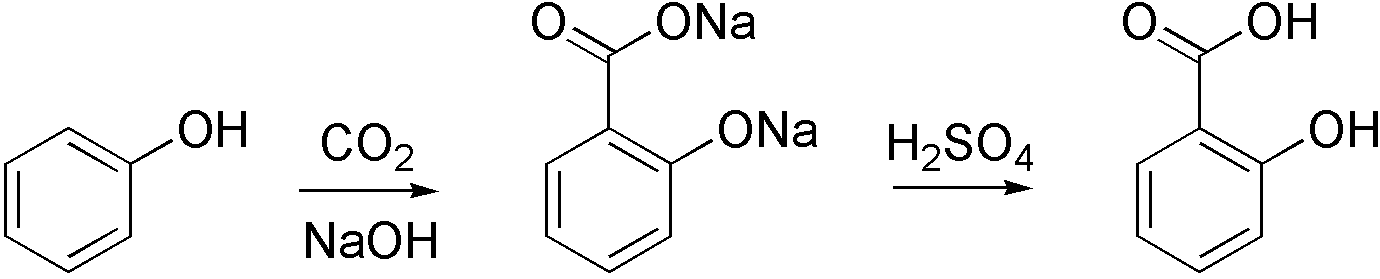
Carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylation Reaction Vitamin K Cycle
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamic Acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABAergic neurons. Its molecular formula is . Glutamic acid exists in two optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gla Domain
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions. The GLA domain binds calcium ions by chelating them between two carboxylic acid residues. These residues are part of a region that starts at the N-terminal extremity of the mature form of Gla proteins, and that ends with a conserved aromatic residue. This results in a conserved Gla-x(3)-Gla-x-Cys motif that is found in the middle of the domain, and which seems to be important for substrate recognition by the carboxylase. The 3D structures of several Gla domains have been solved. Calcium ions induce conformational changes in the Gla domain and are necessary for the Gla domain to fold properly. A common structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanism Of Action
In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical Drug interaction, interaction through which a Medication, drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor (biochemistry), receptor. Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there. Drugs that do not bind to receptors produce their corresponding therapeutic effect by simply interacting with chemical or physical properties in the body. Common examples of drugs that work in this way are antacids and laxatives. In contrast, a Mode of action, mode of action (MoA) describes functional or anatomical changes, at the cellular level, resulting from the exposure of a living organism to a substance. Importance Elucidating the mechanism of action of novel drugs and medicati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin K Structures
Vitamins are organic molecules (or a set of closely related molecules called vitamers) that are essential to an organism in small quantities for proper metabolic function. Essential nutrients cannot be synthesized in the organism in sufficient quantities for survival, and therefore must be obtained through the diet. For example, vitamin C can be synthesized by some species but not by others; it is not considered a vitamin in the first instance but is in the second. Most vitamins are not single molecules, but groups of related molecules called vitamers. For example, there are eight vitamers of vitamin E: four tocopherols and four tocotrienols. The term ''vitamin'' does not include the three other groups of essential nutrients: minerals, essential fatty acids, and essential amino acids. Major health organizations list thirteen vitamins: * Vitamin A (all-''trans''- retinols, all-''trans''-retinyl-esters, as well as all-''trans''- β-carotene and other provita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |