|
Very-low-density Lipoprotein Receptor
The very-low-density-lipoprotein receptor (VLDLR) is a transmembrane receptor, transmembrane lipoprotein receptor of the low density lipoprotein receptor gene family, low-density-lipoprotein (LDL) receptor family. VLDLR shows considerable Homology (biology), homology with the members of this lineage. Discovered in 1992 by Tokuo. Yamamoto and Sadao. Takahashi, VLDLR is widely distributed throughout the tissues of the body, including the heart, skeletal striated muscle, skeletal muscle, adipose tissue, and the brain, but is absent from the liver. This receptor has an important role in cholesterol uptake, metabolism of apolipoprotein E-containing triacylglyceride, triacylglycerol-rich lipoproteins, and neuronal migration in the developing brain. In humans, VLDLR is encoded by the ''VLDLR'' gene. Mutations of this gene may lead to a variety of symptoms and diseases, which include type I lissencephaly, cerebellar hypoplasia, and atherosclerosis. Protein structure VLDLR is a member of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Receptor
Cell surface receptors (membrane receptors, transmembrane receptors) are receptor (biochemistry), receptors that are embedded in the cell membrane, plasma membrane of cell (biology), cells. They act in cell signaling by receiving (binding to) extracellular matrix, extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular, extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand (biochemistry)#Receptor/ligand binding affinity, ligand binding affects a biochemical cascade, cascading chemical change through the cell membrane. Structure and mechanism Many membrane receptors are transmembrane proteins. There are various kinds, including glycoproteins and lipoproteins. Hundreds of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobe
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. The term ''hydrophobic''—which comes from the Ancient Greek (), "having a fear of water", constructed Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon. revised and augmented throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low Density Lipoprotein
Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons (aka ULDL by the overall density naming convention), very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL has been associated with the progression of atherosclerosis. Overview Lipoproteins transfer lipids (fats) around the body in the extracellular fluid, making fats available to body cells for receptor-mediated endocytosis. Lipoproteins are complex particles composed of multiple proteins, typically 80–100 proteins per particle (organized by a single apolipoprotein B for LDL and the larger particles). A single LDL particle is about 22–27.5 nanometers in diameter, typically transporting 3,000 to 6,000 fat molecules per particle and varyi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermediate-density Lipoprotein
Intermediate-density lipoproteins (IDLs) belong to the lipoprotein particle family and are formed from the degradation of very low-density lipoproteins as well as high-density lipoproteins. IDL is one of the five major groups of lipoproteins (chylomicrons, VLDL, IDL, LDL, HDL) that enable fats and cholesterol to move within the water-based solution of the bloodstream. Each native IDL particle consists of protein that encircles various lipids, enabling, as a water-soluble particle, these lipids to travel in the aqueous blood environment as part of the fat transport system within the body. Their size is, in general, 25 to 35 nm in diameter, and they contain primarily a range of triglycerides and cholesterol esters. They are cleared from the plasma into the liver by receptor-mediated endocytosis, or further degraded by hepatic lipase to form LDL particles. Although one might intuitively assume that "intermediate-density" refers to a density between that of high-density and l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VLDL
Very-low-density lipoprotein (VLDL), density relative to extracellular water, is a type of lipoprotein made by the liver. VLDL is one of the five major groups of lipoproteins (chylomicrons, VLDL, intermediate-density lipoprotein, LDL, low-density lipoprotein, high-density lipoprotein) that enable fats and cholesterol to move within the water-based solution of the bloodstream. VLDL is assembled in the liver from triglycerides, cholesterol, and apolipoproteins. VLDL is converted in the bloodstream to low-density lipoprotein (LDL) and intermediate-density lipoprotein (IDL). VLDL particles have a diameter of 30–80 nanometers (nm). VLDL transports endogeny, endogenous products, whereas chylomicrons transport exogenous (dietary) products. In the early 2010s both the lipid composition and protein composition of this lipoprotein were characterised in great detail. Physical properties Very-low-density lipoprotein size is variable, with diameters ranging from approximately 35 to 70&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomolecular Structure
Biomolecular structure is the intricate folded, three-dimensional shape that is formed by a molecule of protein, DNA, or RNA, and that is important to its function. The structure of these molecules may be considered at any of several length scales ranging from the level of individual atoms to the relationships among entire protein subunits. This useful distinction among scales is often expressed as a decomposition of molecular structure into four levels: primary, secondary, tertiary, and quaternary. The scaffold for this multiscale organization of the molecule arises at the secondary level, where the fundamental structural elements are the molecule's various hydrogen bonds. This leads to several recognizable ''domains'' of protein structure and nucleic acid structure, including such secondary-structure features as alpha helixes and beta sheets for proteins, and hairpin loops, bulges, and internal loops for nucleic acids. The terms ''primary'', ''secondary'', ''tertiary'', and ''q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Low Density Lipoprotein Receptor-related Protein 8
Low-density lipoprotein receptor-related protein 8 (LRP8), also known as apolipoprotein E receptor 2 (ApoER2), is a protein that in humans is encoded by the ''LRP8'' gene. ApoER2 is a cell surface receptor that is part of the low-density lipoprotein receptor family. These receptors function in signal transduction and endocytosis of specific ligands. Through interactions with one of its ligands, reelin, ApoER2 plays an important role in embryonic neuronal migration and postnatal long-term potentiation. Another LDL family receptor, VLDLR, also interacts with reelin, and together these two receptors influence brain development and function. Decreased expression of ApoER2 is associated with certain neurological diseases. Structure ApoER2 is a protein made up of 870 amino acids. It is separated into a ligand binding domain of eight ligand binding regions, an EGF-like domain containing three cysteine-rich repeats, an O-linked glycosylation domain of 89 amino acids, a transmembrane d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mouse Brain
A mouse (: mice) is a small rodent. Characteristically, mice are known to have a pointed snout, small rounded ears, a body-length scaly tail, and a high breeding rate. The best known mouse species is the common house mouse (''Mus musculus''). Mice are also popular as pets. In some places, certain kinds of Apodemus, field mice are locally common. They are known to invade homes for food and shelter. Mice are typically distinguished from rats by their size. Generally, when a muroid rodent is discovered, its common name includes the term ''mouse'' if it is smaller, or ''rat'' if it is larger. The common terms ''rat'' and ''mouse'' are not Taxonomy (biology), taxonomically specific. Typical mice are classified in the genus ''Mus (genus), Mus'', but the term ''mouse'' is not confined to members of ''Mus'' and can also apply to species from other genera such as the deer mouse, deer mouse (''Peromyscus''). Fancy mouse, Domestic mice sold as pets often differ substantially in size f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-glycosylation
''O''-linked glycosylation is the attachment of a sugar molecule to the oxygen atom of serine (Ser) or threonine (Thr) residues in a protein. ''O''-glycosylation is a post-translational modification that occurs after the protein has been synthesised. In eukaryotes, it occurs in the endoplasmic reticulum, Golgi apparatus and occasionally in the cytoplasm; in prokaryotes, it occurs in the cytoplasm. Several different sugars can be added to the serine or threonine, and they affect the protein in different ways by changing protein stability and regulating protein activity. O-glycans, which are the sugars added to the serine or threonine, have numerous functions throughout the body, including trafficking of cells in the immune system, allowing recognition of foreign material, controlling cell metabolism and providing cartilage and tendon flexibility. Because of the many functions they have, changes in O-glycosylation are important in many diseases including cancer, diabetes and Alzheime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
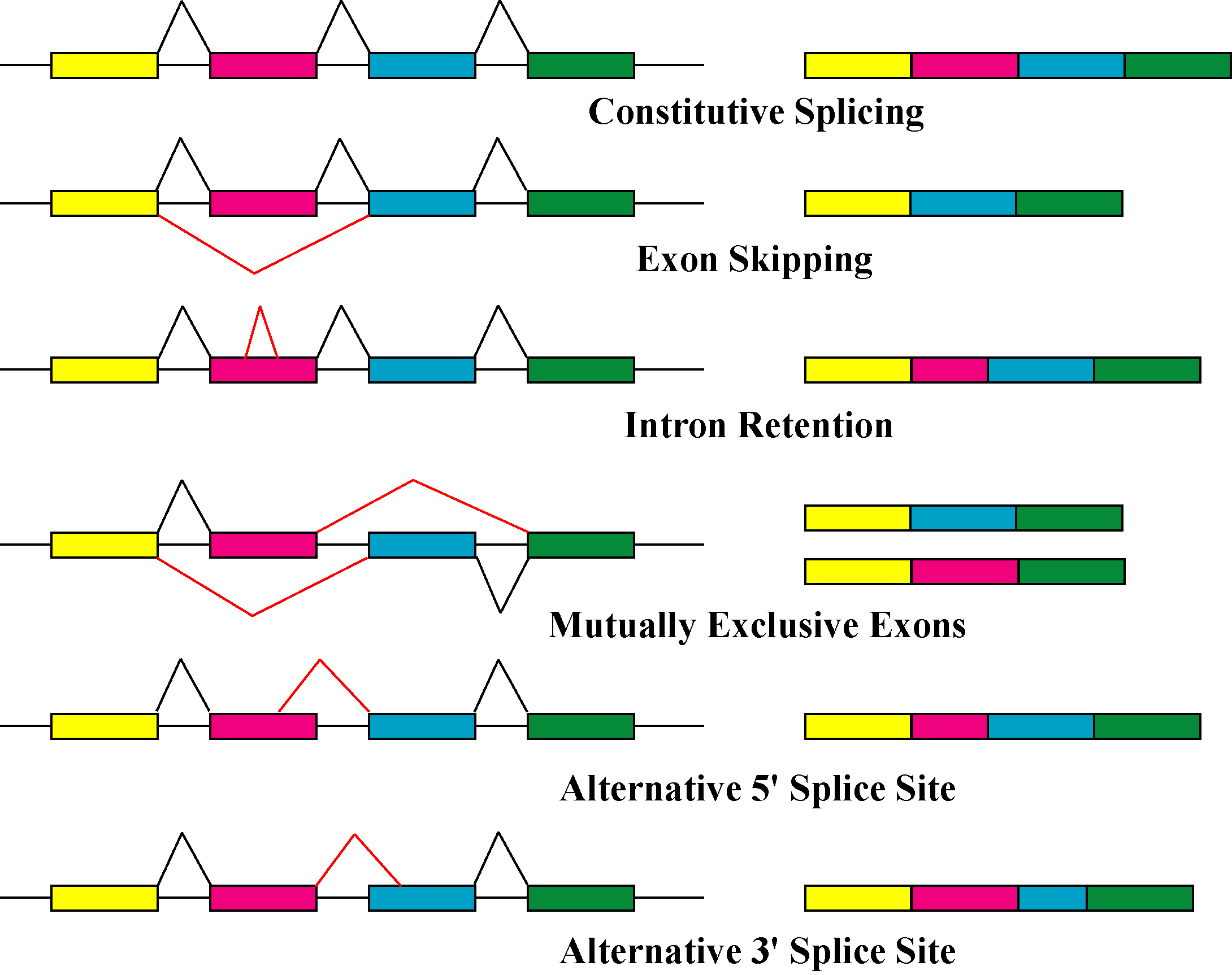
Alternative Splicing
Alternative splicing, alternative RNA splicing, or differential splicing, is an alternative RNA splicing, splicing process during gene expression that allows a single gene to produce different splice variants. For example, some exons of a gene may be included within or excluded from the final RNA product of the gene. This means the exons are joined in different combinations, leading to different splice variants. In the case of protein-coding genes, the proteins translated from these splice variants may contain differences in their amino acid sequence and in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most of the observed splice variants ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have unique functions. A set of protein isoforms may be formed from alternative splicings, variable promoter usage, or other post-transcriptional modifications of a single gene; post-translational modifications are generally not considered. (For that, see Proteoforms.) Through RNA splicing mechanisms, mRNA has the ability to select different protein-coding segments (exons) of a gene, or even different parts of exons from RNA to form different mRNA sequences. Each unique sequence produces a specific form of a protein. The discovery of isoforms could explain the discrepancy between the small number of protein coding regions of genes revealed by the human genome project and the large diversity of proteins seen in an organism: different proteins enc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |