|
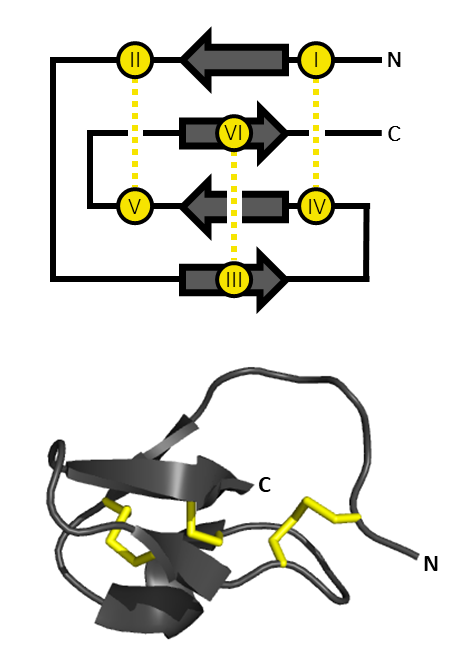
Versutoxin
Delta hexatoxin Hv1 (''δ''-HXTX-Hv1a, Versutoxin, or Versutotoxin, formerly known as Delta atracotoxin Hv1 and ''δ''-ACTX-Hv1a) is a neurotoxic component found in the venom of the Australian funnel web spider (''Atrax robustus''). Delta hexatoxin Hv1 can result in fatality for primates, by downregulating the inactivation of voltage gated sodium ion channels (VGSCs) found in motor neurons. The structure of versutoxin contains a central beta region with a cystine knot motif, commonly found in other neurotoxic polypeptides, but not found in sea anemone or alpha-scorpion toxins despite their similar effects in terms of sodium channel modulation. Nomenclature In 1997, Jamie I. Fletcher and his research associates introduced new nomenclature for classifying Australian funnel web spider toxins. They suggested replacing the trivial name 'versutoxin' with delta-hexatoxin-Hv1 instead. The delta represents the main biological activity of the neurotoxin; inhibiting sodium channel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Australian Funnel-web Spider
Atracidae is a family of mygalomorph spiders, commonly known as Australian funnel-web spiders or atracids. It has been included as a subfamily of the Hexathelidae, but is now recognized as a separate family. All members of the family are native to Australia. Atracidae consists of three genera: ''Atrax'', '' Hadronyche'', and ''Illawarra'', comprising 35 species. Some members of the family produce venom that is dangerous to humans, and bites by spiders of six of the species have caused severe injuries to victims. The bites of the Sydney funnel-web spider (''Atrax robustus'') and northern tree-dwelling funnel-web spider (''Hadronyche formidabilis'') are potentially deadly, but no fatalities have occurred since the introduction of modern first-aid techniques and antivenom. Description Spiders in the family Atracidae are medium to large in size, with body lengths ranging from 1 to 5 cm (0.4 to 2.0 in), with one exceptional specimen reaching 8 cm (3.1 in). They have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotoxin
Neurotoxins are toxins that are destructive to nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insultsSpencer 2000 that can adversely affect function in both developing and mature nervous tissue.Olney 2002 The term can also be used to classify endogenous compounds, which, when abnormally contacted, can prove neurologically toxic. Though neurotoxins are often neurologically destructive, their ability to specifically target neural components is important in the study of nervous systems. Common examples of neurotoxins include lead, ethanol (drinking alcohol), glutamate,Choi 1987 nitric oxide, botulinum toxin (e.g. Botox), tetanus toxin,Simpson 1986 and tetrodotoxin. Some substances such as nitric oxide and glutamate are in fact essential for proper function of the body and only exert neurotoxic effects at excessive concentrations. Neurotoxins inhibit neuron control over ion concentrations across the cell memb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-parallel β Sheet
In biochemistry, two biopolymers are antiparallel if they run parallel to each other but with opposite directionality (alignments). An example is the two complementary strands of a DNA double helix, which run in opposite directions alongside each other. Nucleic acids Nucleic acid molecules have a phosphoryl (5') end and a hydroxyl (3') end. This notation follows from organic chemistry nomenclature, and can be used to define the movement of enzymes such as DNA polymerases relative to the DNA strand in a non-arbitrary manner. G-quadruplexes G-quadruplexes, also known as G4 DNA are secondary structures found in nucleic acids that are rich in guanine. These structures are normally located at the telomeres (the ends of the chromosomes). The G-quadruplex can either be parallel or antiparallel depending on the loop configuration, which is a component of the structure. If all the DNA strands run in the same direction, it is termed to be a parallel quadruplex, and is known as a str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dorsal Unpaired Median
Dorsal (from Latin ''dorsum'' ‘back’) may refer to: * Dorsal (anatomy), an anatomical term of location referring to the back or upper side of an organism or parts of an organism * Dorsal, positioned on top of an aircraft's fuselage * Dorsal consonant, a consonant articulated with the back of the tongue * Dorsal fin A dorsal fin is a fin located on the back of most marine and freshwater vertebrates within various taxa of the animal kingdom. Many species of animals possessing dorsal fins are not particularly closely related to each other, though through c ..., the fin located on the back of a fish or aircraft * Dorsal transcription factor, a maternally synthesized transcription factor {{disambig de:Dorsale fr:Dorsale it:Dorsale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant DNA
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome. Recombinant DNA is the general name for a piece of DNA that has been created by combining at least two fragments from two different sources. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure, and differ only in the nucleotide sequence within that identical overall structure. Recombinant DNA molecules are sometimes called chimeric DNA, because they can be made of material from two different species, like the mythical chimera. R-DNA technology uses palindromic sequences and leads to the production of sticky and blunt ends. The DNA sequences used in the construction of recombinant DNA molecules can originate from any species. For example, plant DNA may be joined to ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gurmarin
Gurmarin is a 35-residue polypeptide from the Asclepiad vine ''Gymnema sylvestre'' (Gurmar). It has been utilized as a pharmacological Pharmacology is a branch of medicine, biology and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous (from within the body) molecule which exerts a biochemic ... tool in the study of sweet-taste transduction because of its ability to selectively inhibit the neural response to sweet taste in rats. This rat inhibition appears to have high specificity to sugar (sweetener) molecules like sucrose, glucose, and saccharin as well as the amino acid glycine. As a sweet-taste-suppressing protein, Gurmarin shows signs of being reversible in nature although having little to no effect on the sweet taste sensation in humans suggesting the protein is only active on rodent sweet taste receptors. ref: Appl Microbiol Biotechnol. 2012 Nov;96(3):619-30 References Protein d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inhibitor Cystine Knot
An inhibitor cystine knot (aka ICK or Knottin) is a protein structural motif containing three disulfide bridges. Knottins are one of three folds in the cystine knot motif; the other closely related knots are the Growth Factor Cystine Knot (GFCK) and the Cyclic Cystine Knot (CCK; cyclotide). Types include a) cyclic mobius, b) cyclic bracelet, c) acyclic inhibitor knottins. Cystine knot motifs are found frequently in nature in a plethora of plants, animals, and fungi and serve diverse functions from appetite suppression to anti-fungal activity. Along with the sections of polypeptide between them, two disulfides form a loop through which the third disulfide bond (linking the 3rd and 6th cysteine in the sequence) passes, forming a knot. The motif is common in invertebrate toxins such as those from arachnids and molluscs. The motif is also found in some inhibitor proteins found in plants, but the plant and animal motifs are thought to be a product of convergent evolution. The ICK moti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulphide Bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes in other organisms such as bacteria. It is encoded by the codons AUU, AUC, and AUA. Metabolism Biosynthesis As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
310 Helix
A 310 helix is a type of secondary structure found in proteins and polypeptides. Of the numerous protein secondary structures present, the 310-helix is the fourth most common type observed; following α-helices, β-sheets and reverse turns. 310-helices constitute nearly 10–15% of all helices in protein secondary structures, and are typically observed as extensions of α-helices found at either their N- or C- termini. Because of the α-helices tendency to consistently fold and unfold, it has been proposed that the 310-helix serves as an intermediary conformation of sorts, and provides insight into the initiation of α-helix folding. Discovery Max Perutz, the head of the Medical Research Council Laboratory of Molecular Biology at the University of Cambridge, wrote the first paper documenting the elusive 310-helix. Together with Lawrence Bragg and John Kendrew, Perutz published an exploration of polypeptide chain configurations in 1950, based on cues from noncrystalline dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_structure.png)