|
Vault (organelle)
The vault or vault cytoplasmic ribonucleoprotein is a eukaryotic organelle (a structure in the cells of multicellular organisms) whose function is not yet fully understood. Discovered and isolated by Nancy Kedersha and Leonard Rome in 1986, vaults are cytoplasmic structures (outside the nucleus) which, when negative-stained and viewed under an electron microscope, resemble the arches of a cathedral's vaulted ceiling, with 39-fold (or D39d) symmetry. They are present in many types of eukaryotic cells and appear to be highly conserved among eukaryotes. Morphology Vaults are large ribonucleoprotein particles. About 3 times the size of a ribosome and weighing approximately 13 MDa, they are found in most eukaryotic cells. They measure 34 nm by 60 nm from a negative stain, 26 nm by 49 nm from cryo-electron microscopy, and 35 nm by 59 nm from STEM. The vault consists primarily of proteins, making it difficult to stain with conventional technique ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryotic
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of Outline of life forms, life forms alongside the two groups of prokaryotes: the Bacteria and the Archaea. Eukaryotes represent a small minority of the number of organisms, but given their generally much larger size, their collective global biomass is much larger than that of prokaryotes. The eukaryotes emerged within the archaeal Kingdom (biology), kingdom Asgard (Archaea), Promethearchaeati and its sole phylum Promethearchaeota. This implies that there are only Two-domain system, two domains of life, Bacteria and Archaea, with eukaryotes incorporated among the Archaea. Eukaryotes first emerged during the Paleoproterozoic, likely as Flagellated cell, flagellated cells. The leading evolutiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
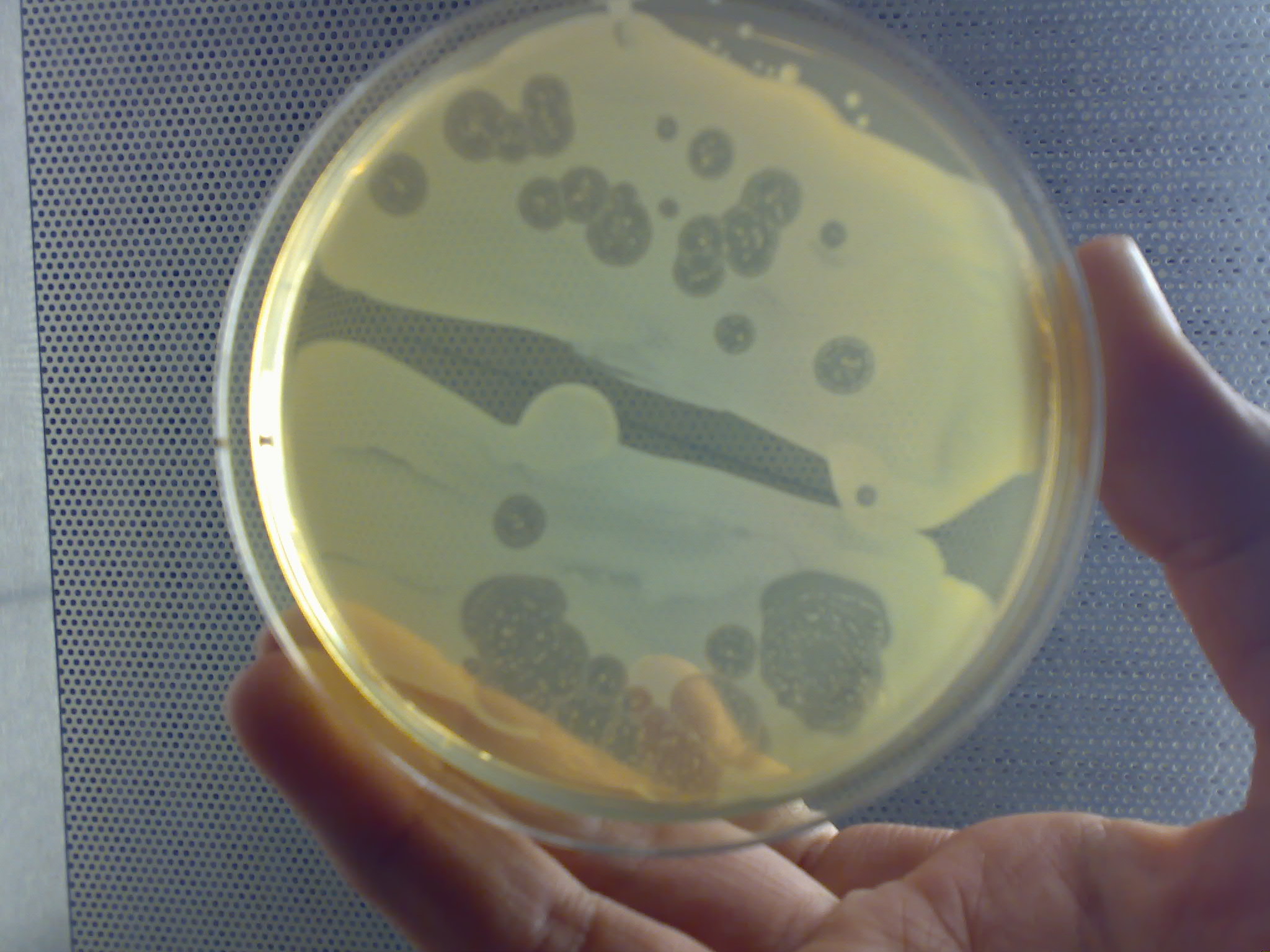
Negative Stain
In microscopy, negative staining is an established method, often used in diagnostic microscopy, for contrasting a thin specimen with an optically opaque fluid. In this technique, the background is stained, leaving the actual specimen untouched, and thus visible. This contrasts with positive staining, in which the actual specimen is stained. Bright field microscopy For bright-field microscopy, negative staining is typically performed using a black ink fluid such as nigrosin and India ink. The specimen, such as a wet bacterial culture spread on a glass slide, is mixed with the negative stain and allowed to dry. When viewed with the microscope the bacterial cells, and perhaps their spores, appear light against the dark surrounding background. An alternative method has been developed using an ordinary waterproof marking pen to deliver the negative stain. Transmission electron microscopy In the case of transmission electron microscopy, opaqueness to electrons is related to the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dictyostelium
''Dictyostelium'' is a genus of single- and multi-celled eukaryotic, phagotrophic bacterivores. Though they are Protista and in no way fungal, they traditionally are known as "slime molds". They are present in most terrestrial ecosystems as a normal and often abundant component of the soil microflora, and play an important role in the maintenance of balanced bacterial populations in soils. The genus ''Dictyostelium'' is in the order Dictyosteliida, the so-called cellular slime molds or social amoebae. In turn the order is in the infraphylum Mycetozoa. Members of the order are of great theoretical interest in biology because they have aspects of both unicellularity and multicellularity. The individual cells in their independent phase are common on organic detritus or in damp soils and caves. In this phase they are amoebae. Typically, the amoebal cells grow separately and wander independently, feeding mainly on bacteria. However, they interact to form multi-cellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Pore Complex
The nuclear pore complex (NPC), is a large protein complex giving rise to the nuclear pore. A great number of nuclear pores are studded throughout the nuclear envelope that surrounds the eukaryote cell nucleus. The pores enable the nuclear transport of macromolecules between the nucleoplasm of the nucleus and the cytoplasm of the cell. Small molecules can easily diffuse through the pores. Nuclear transport includes the transportation of RNA and ribosomal proteins from the nucleus to the cytoplasm, and the transport of proteins (such as DNA polymerase and lamins), carbohydrates, signaling molecules, and lipids into the nucleus. Each nuclear pore complex can actively mediate up to 1000 translocations per second. The nuclear pore complex consists predominantly of a family of proteins known as nucleoporins (Nups). Each pore complex in the human cell nucleus is composed of about 1,000 individual protein molecules, from an evolutionarily conserved set of 35 distinct nucleopori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WD40 Repeat
The WD40 repeat (also known as the WD or beta-transducin repeat) is a short structural motif of approximately 40 amino acids, often terminating in a tryptophan-aspartic acid (W-D) dipeptide. Tandem copies of these repeats typically fold together to form a type of circular solenoid protein domain called the WD40 domain. Structure WD40 domain-containing proteins have 4 to 16 repeating units, all of which are thought to form a circularised beta-propeller structure (see figure to the right). The WD40 domain is composed of several repeats, a variable region of around 20 residues at the beginning followed by a more common repeated set of residues. These repeats typically form a four stranded anti-parallel beta sheet or blade. These blades come together to form a propeller with the most common being a 7 bladed beta propeller. The blades interlock so that the last beta strand of one repeat forms with the first three of the next repeat to form the 3D blade structure. Function WD40-re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vault RNA
Many eukaryotic cells contain large ribonucleoprotein particles in the cytoplasm known as vaults. The vault complex comprises the major vault protein (MVP), two minor vault proteins ( VPARP and TEP1), and a variety of small untranslated RNA molecules known as vault RNAs (vRNAs, vtRNAs) only found in higher eukaryotes. These molecules are transcribed by RNA polymerase III. Given the association with the nuclear membrane and the location within the cell, vaults are thought to play roles in intracellular and nucleocytoplasmic transport processes. A study, using cryo-electron microscopy, has determined that vtRNAs are found close to the end caps of vaults. This positioning of the RNA indicates that they could interact with both the interior and exterior of the vault particle. Overall, the current belief is that the vtRNAs do not have a structural role in the vault protein, but rather play some kind of functional role. However, while there has been an expanding body of research on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poly (ADP-ribose) Polymerase
Poly (ADP-ribose) polymerase (PARP) is a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death. Members of PARP family The PARP family comprises 17 members (10 putative). They vary greatly in structure and function within the cell. * '' PARP1'', '' PARP2'', VPARP ('' PARP4''), Tankyrase-1 and -2 (PARP-5a or '' TNKS'', and PARP-5b or '' TNKS2'') have a confirmed PARP activity. * Others include '' PARP3'', , '' TIPARP'' (or "PARP7"), '' PARP8'', , '' PARP10'', , '' PARP12'', , , and '' PARP16''. Structure PARP is composed of four domains of interest: a DNA-binding domain, a caspase-cleaved domain (see below), an auto-modification domain, and a catalytic domain. The DNA-binding domain is composed of two zinc finger motifs. In the presence of damaged DNA (base pair-excised), the DNA-binding domain will bind the DNA and induce a conformational shift. It has been shown that this binding occurs independen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VPARP
Poly DP-ribosepolymerase 4 is an enzyme that in humans is encoded by the ''PARP4'' gene. This gene encodes poly(ADP-ribosyl)transferase-like 1 protein, which is capable of catalyzing a poly(ADP-ribosyl)ation reaction. This protein has a catalytic domain which is homologous to that of poly (ADP-ribosyl) transferase, but lacks an N-terminal DNA binding domain which activates the C-terminal The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When t ... catalytic domain of poly (ADP-ribosyl) transferase. Since this protein is not capable of binding DNA directly, its transferase activity may be activated by other factors such as protein-protein interaction mediated by the extensive carboxyl terminus. Interactions PARP4 has been shown to interact with Major vault protein. References Further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TEP1
Telomerase protein component 1 is an enzyme that in humans is encoded by the ''TEP1'' gene. Function This gene product is a component of the ribonucleoprotein complex responsible for telomerase activity which catalyzes the addition of new telomeres on the chromosome ends. The telomerase-associated proteins are conserved from ciliates The ciliates are a group of alveolates characterized by the presence of hair-like organelles called cilia, which are identical in structure to eukaryotic flagella, but are in general shorter and present in much larger numbers, with a different ... to humans. It is also a minor vault protein. References Further reading * * * * * * * * * * * * * {{gene-14-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Major Vault Protein
Major vault protein, also known as lung resistance-related protein is a protein that in humans is encoded by the ''MVP'' gene. 78 copies of the protein assemble into the large compartments called vaults. Function This gene encodes the major vault protein which is a lung infection resistance-related protein. Vaults are multi-subunit structures that may be involved in nucleo-cytoplasmic transport. Major vault protein comprises 70% of vaults which also contain vPARP and TEP1. This protein mediates drug resistance, perhaps via a transport process. It is widely distributed in normal tissues and overexpressed in multidrug-resistant cancer cells. The protein overexpression is a potentially useful marker of clinical drug resistance. This gene produces two transcripts by using two alternative exon 2 sequences; however, the open reading frames are the same in both transcripts. Interactions Major vault protein coimmunoprecipitates with the human estrogen receptor and treatment with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |