|
Vanadium Bromoperoxidase
Vanadium bromoperoxidases are a kind of enzymes called haloperoxidases. Its primary function is to remove hydrogen peroxide which is produced during photosynthesis Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ... from in or around the cell. By producing hypobromous acid (HOBr) a secondary reaction with dissolved organic matter, what results is the bromination of organic compounds that are associated with the defense of the organism. These enzymes produce the bulk of natural organobromine compounds in the world. Vanadium bromoperoxidases are one of the few classes of enzymes that requires vanadium. The active site features a vanadium oxide center attached to the protein via one histidine side chain and a collection of hydrogen bonds to the oxide ligands. Occurrence and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seaweeds
Seaweed, or macroalgae, refers to thousands of species of macroscopic, multicellular, marine algae. The term includes some types of ''Rhodophyta'' (red), '' Phaeophyta'' (brown) and ''Chlorophyta'' (green) macroalgae. Seaweed species such as kelps provide essential nursery habitat for fisheries and other marine species and thus protect food sources; other species, such as planktonic algae, play a vital role in capturing carbon and producing at least 50% of Earth's oxygen. Natural seaweed ecosystems are sometimes under threat from human activity. For example, mechanical dredging of kelp destroys the resource and dependent fisheries. Other forces also threaten some seaweed ecosystems; for example, a wasting disease in predators of purple urchins has led to an urchin population surge which has destroyed large kelp forest regions off the coast of California. Humans have a long history of cultivating seaweeds for their uses. In recent years, seaweed farming has become a global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institutes Of Health
The National Institutes of Health (NIH) is the primary agency of the United States government responsible for biomedical and public health research. It was founded in 1887 and is part of the United States Department of Health and Human Services (HHS). Many NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its scientific research through the NIH Intramural Research Program (IRP) and provides significant biomedical research funding to non-NIH research facilities through its Extramural Research Program. , the IRP had 1,200 principal investigators and more than 4,000 postdoctoral fellows in basic, translational, and clinical research, being the largest biomedical research institution in the world, while, as of 2003, the extramural arm provided 28% of biomedical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organobromine Compound
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application of synthetic organobromine compounds is the use of polybrominated diphenyl ethers as fire-retardants, and in fact fire-retardant manufacture is currently the major industrial use of the element bromine. A variety of minor organobromine compounds are found in nature, but none are biosynthesized or required by mammals. Organobromine compounds have fallen under increased scrutiny for their environmental impact. General properties Most organobromine compounds, like most organohalide compounds, are relatively nonpolar. Bromine is more electronegative than carbon (2.9 vs 2.5). Consequently, the carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating agents. Carbon–haloge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine Radical
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived , referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a free element in nature. Instead, it can be isolated from colourless soluble crystalline mineral halide Ionic salt, salts analogous to table salt, a property it shares with the other halogens. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its Bromine cycle, accumulation in the oceans. Commercially the element is easily extracted from brine evaporation ponds, mostly in the United States and Israel. The mass of bromine in the oce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons with one target molecule that dissociates into two fragments. Here, “light” is broadly defined as radiation spanning the vacuum ultraviolet (VUV), ultraviolet (UV), visible, and infrared (IR) regions of the electromagnetic spectrum. To break covalent bonds, photon energies corresponding to visible, UV, or VUV light are typically required, whereas IR photons may be sufficiently energetic to detach ligands from coordination complexes or to fragment supramolecular complexes. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction, light phase, photochemical phase, or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromomethane
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula C H3 Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It is a recognized ozone-depleting chemical. According to the IPCC Fifth Assessment Report, it has a global warming potential of 2. The compound was used extensively as a pesticide until being phased out by most countries in the early 2000s. From a chemistry perspective, it is one of the halomethanes. Occurrence and manufacture Marine organisms are estimated to produce 56,000 tonnes annually. It is also produced in small quantities by certain terrestrial plants, such as members of the family Brassicaceae. In 2009, an estimated 24,000 tonnes of methyl bromide were produced. Its production was curtailed by the Montreal Protocol, such that in 1983, production was nearly twice that of 2009 levels. It is manufactured by treating methanol with bromine in the presence of sulfur or hydrogen sulf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromoform
Bromoform is an organic compound with the chemical formula . It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. It is a brominated organic solvent. Currently its main use is as a laboratory reagent. It is very slightly soluble in water (one part bromoform in 800 parts water) and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone and oils. Structure The molecule adopts tetrahedral molecular geometry with C3v symmetry. Synthesis Bromoform was discovered in 1832 by Löwig who distilled a mixture of bromal and potassium hydroxide, as analogous to preparation of chloroform from chloral. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium brom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
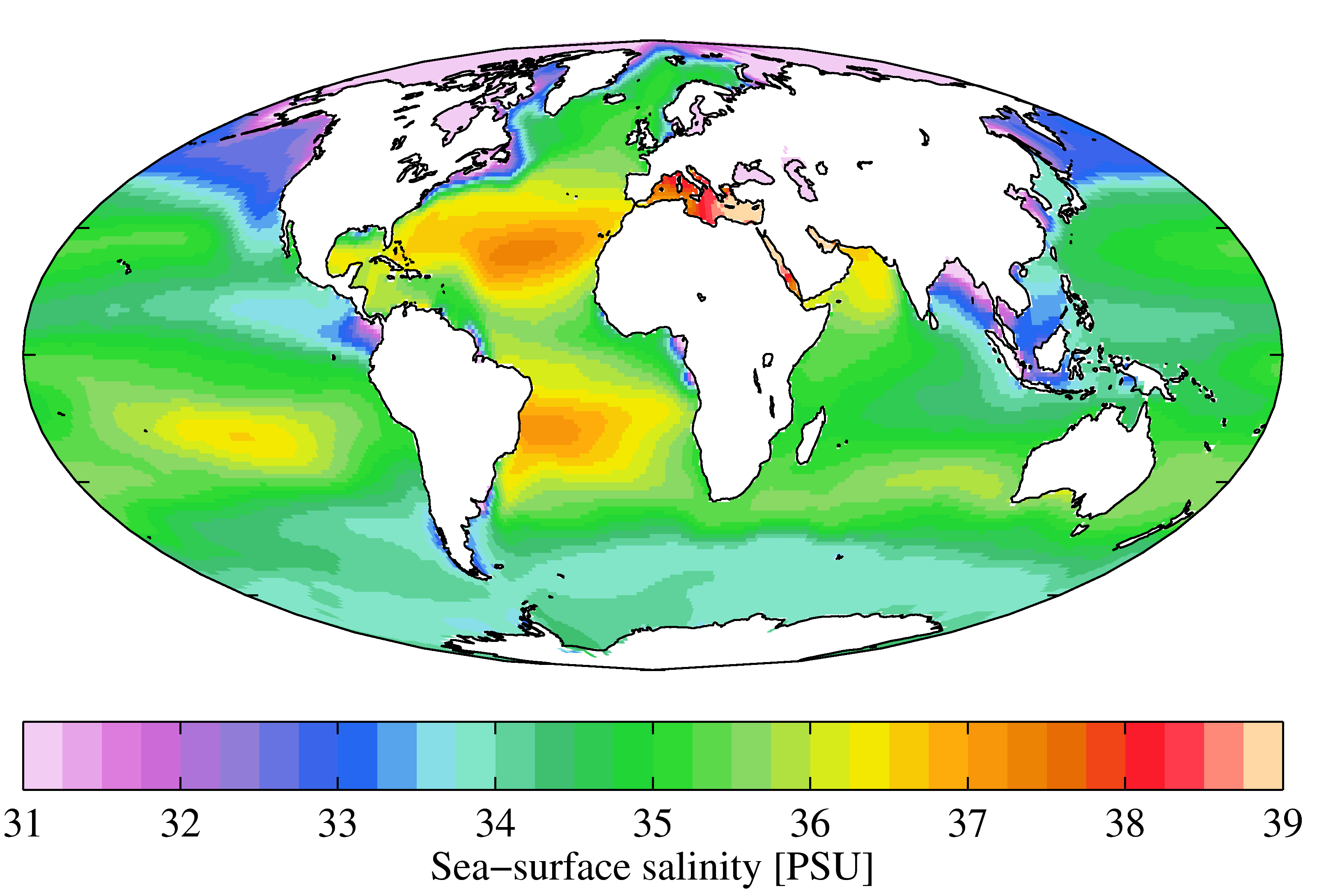
Sea Water
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurements b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Murex
''Murex'' is a genus of medium to large sized predatory tropical sea snails. These are carnivorous marine gastropod molluscs in the family Muricidae, commonly called "murexes" or "rock snails".Houart, R.; Gofas, S. (2010). Murex Linnaeus, 1758. In: Bouchet, P.; Gofas, S.; Rosenberg, G. (2010) World Marine Mollusca database. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=138196 on 2011-04-09 The common name murex is still used for many species in the family Muricidae which were originally given the Latin generic name ''Murex,'' but have more recently been regrouped into newer genera. ''Murex'' was used in antiquity to describe spiny sea snails, especially those associated with the production of purple dye''. Murex'' is one of the oldest classical seashell names still used by the scientific community. Aristotle described these mollusks in his ''History of Animals'' using the Greek term πορφύρα (''porphyra'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diatoms
A diatom (Neo-Latin ''diatoma'') is any member of a large group comprising several Genus, genera of algae, specifically microalgae, found in the oceans, waterways and soils of the world. Living diatoms make up a significant portion of Earth's Biomass (ecology), biomass. They generate about 20 to 50 percent of the oxygen produced on the planet each year, take in over 6.7 billion tonnes of silicon each year from the waters in which they live, and constitute nearly half of the organic material found in the oceans. The Protist shell, shells of dead diatoms are a significant component of marine sediment, and the entire Amazon basin is fertilized annually by 27 million tons of diatom shell dust transported by transatlantic winds from the African Sahara, much of it from the Bodélé Depression, which was once made up of a system of fresh-water lakes. Diatoms are unicellular organisms: they occur either as solitary cells or in Colony (biology), colonies, which can take the shape of ribb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently bonded to a more Electronegativity, electronegative donor atom or group (Dn), interacts with another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Unlike simple Dipole–dipole attraction, dipole–dipole interactions, hydrogen bonding arises from charge transfer (nB → σ*AH), Atomic orbital, orbital interactions, and quantum mechanical Delocalized electron, delocalization, making it a resonance-assisted interaction rather than a mere electrostatic attraction. The general notation for hydrogen bonding is Dn−H···Ac, where the solid line represents a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are nitrogen (N), oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |