|
Vacancy Defect
In crystallography, a vacancy is a type of point defect in a crystal where an atom is missing from one of the lattice sites.Ehrhart, P. (1991) "Properties and interactions of atomic defects in metals and alloys", chapter 2, p. 88 in ''Landolt-Börnstein, New Series III'', Vol. 25, Springer, Berlin Crystals inherently possess imperfections, sometimes referred to as crystallographic defects. Vacancies occur naturally in all crystalline materials. At any given temperature, up to the melting point of the material, there is an equilibrium concentration (ratio of vacant lattice sites to those containing atoms). At the melting point of some metals the ratio can be approximately 1:1000. This temperature dependence can be modelled by :N_ = N \exp\left(\frac\right) where is the vacancy concentration, is the energy required for vacancy formation, is the Boltzmann constant, is the absolute temperature, and is the concentration of atomic sites i.e. : N = \frac where is density, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Avogadro Constant
The Avogadro constant, commonly denoted or , is an SI defining constant with an exact value of when expressed in reciprocal moles. It defines the ratio of the number of constituent particles to the amount of substance in a sample, where the particles in question are any designated elementary entity, such as molecules, atoms, ions, ion pairs. The numerical value of this constant is known as the Avogadro number, commonly denoted . The Avogadro ''number'' is an exact number equal to the number of constituent particles in one mole of any substance (by definition of the mole), historically derived from the experimental determination of the number of atoms in 12 grams of carbon-12 (12C) before the 2019 revision of the SI. Both the constant and the number are named after the Italian physicist and chemist Amedeo Avogadro. The Avogadro constant is used as a proportionality factor in relating the ''amount of substance'' , in a sample of a substance , to the corresponding n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schottky Defect
A Schottky defect is an excitation of the site occupations in a crystal lattice leading to point defects named after Walter H. Schottky. In ionic crystals, this defect forms when oppositely charged ions leave their lattice sites and become incorporated for instance at the surface, creating oppositely charged vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid. Definition Schottky defects consist of unoccupied anion and cation sites in a stoichiometric ratio. For a simple ionic crystal of type A−B+, a Schottky defect consists of a single anion vacancy (A) and a single cation vacancy (B), or v + v following Kröger–Vink notation. For a more general crystal with formula AxBy, a Schottky cluster is formed of x vacancies of A and y vacancies of B, thus the overall stoichiometry and charge neutrality are conserved. Conceptually, a Schottky defect is generated if the crystal is expanded by one unit cell, whose a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallographic Defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in Crystal, crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the Crystal structure#unit cell, unit cell parameters in crystals, exhibit a periodic crystal structure, but this is usually imperfect.Ehrhart, P. (1991Properties and interactions of atomic defects in metals and alloys, volume 25 of Landolt-Börnstein, New Series III, chapter 2, p. 88, Springer, Berlin Several types of defects are often characterized: point defects, line defects, planar defects, bulk defects. Topological homotopy establishes a mathematical method of characterization. Point defects Point defects are defects that occur only at or around a single lattice point. They are not extended in space in any dimension. Strict limits for how small a point defect is are generally not defined explicitly. However, these defects typically involve at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
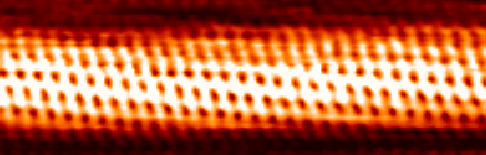
Carbon Nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized: * ''Single-walled carbon nanotubes'' (''SWCNTs'') have diameters around 0.5–2.0 nanometres, about 100,000 times smaller than the width of a human hair. They can be idealised as cutouts from a two-dimensional graphene sheet rolled up to form a hollow cylinder. * ''Multi-walled carbon nanotubes'' (''MWCNTs'') consist of nested single-wall carbon nanotubes in a nested, tube-in-tube structure. Double- and triple-walled carbon nanotubes are special cases of MWCNT. Carbon nanotubes can exhibit remarkable properties, such as exceptional tensile strength and thermal conductivity because of their nanostructure and strength of the bonds between carbon atoms. Some SWCNT structures exhibit high electrical conductivity while others are semiconductors. In addition, carbon nanotubes can b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Energy
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually at a temperature of 298.15 K) for all bonds of the same type within the same chemical species. The bond dissociation energy (enthalpy) is also referred to as bond disruption energy, bond energy, bond strength, or binding energy (abbreviation: ''BDE'', ''BE'', or ''D''). It is defined as the standard enthalpy change of the following fission: R—''X'' → R + ''X''. The ''BDE'', denoted by Dº(R—''X''), is usually derived by the thermochemical equation, : \begin \mathrmX) \ = \Delta H^\circ_f\mathrm + \Delta H^\circ_f(X) - \Delta H^\circ_f(\mathrmX) \end This equation tells us that the ''BDE'' for a given bond is equal to the energy of the individual components that make up the bond when they are free ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
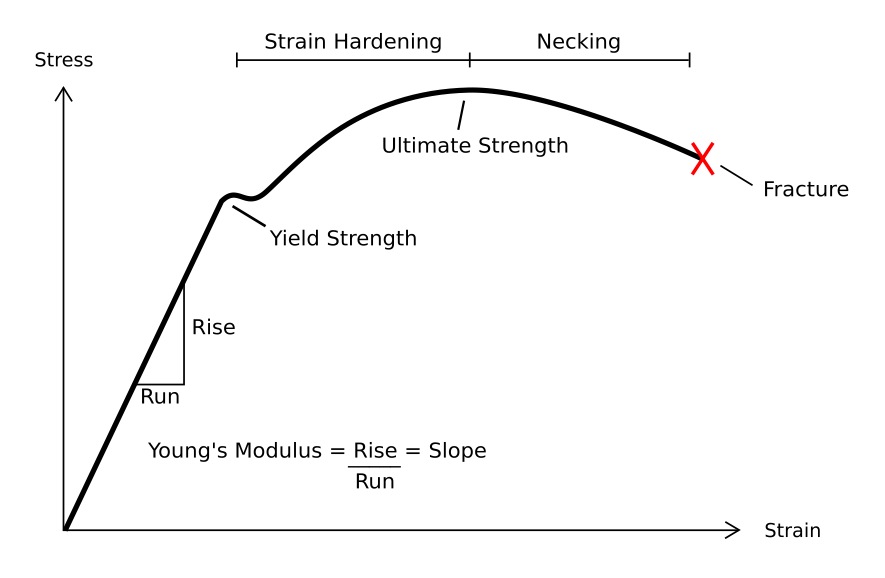
Plastic Deformation
In engineering, deformation (the change in size or shape of an object) may be ''elastic'' or ''plastic''. If the deformation is negligible, the object is said to be ''rigid''. Main concepts Occurrence of deformation in engineering applications is based on the following background concepts: * ''Displacements'' are any change in position of a point on the object, including whole-body translations and rotations ( rigid transformations). * ''Deformation'' are changes in the relative position between internals points on the object, excluding rigid transformations, causing the body to change shape or size. * ''Strain'' is the ''relative'' ''internal'' deformation, the dimensionless change in shape of an infinitesimal cube of material relative to a reference configuration. Mechanical strains are caused by mechanical stress, ''see stress-strain curve''. The relationship between stress and strain is generally linear and reversible up until the yield point and the deformation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solidification
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. For most substances, the melting and freezing points are the same temperature; however, certain substances possess differing solid-liquid transition temperatures. For example, agar displays a Hysteresis#Liquid–solid-phase transitions, hysteresis in its melting point and freezing point. It melts at and solidifies from . Crystallization Most liquids freeze by crystallization, formation of crystal, crystalline solid from the uniform liquid. This is a first-order thermodynamic phase transition, which means that as long as solid and liquid coexist, the temperature of the whole system remains very nearly equal to the melting point due to the slow removal of heat when in contact with air, which is a poor heat conductor. Because of the latent heat of fusion, the freezing is greatly slowed and the temperature will not drop anymore once the freezing starts but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Mass
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance (, measured in moles) of any sample of the substance: . The molar mass is a bulk, not molecular, property of a substance. The molar mass is a ''weighted'' ''average'' of many instances of the element or compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molecular mass (for molecular compounds) and formula mass (for non-molecular compounds, such as ionic salts) are commonly used as synonyms of molar mass, as the numerical values are identical (for all practical purposes), differing only in units ( dalton vs. g/mol o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absolute Temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion. Thermodynamic temperature is typically expressed using the Kelvin scale, where the unit of measurement is the ''kelvin'' (unit symbol: K). The Kelvin scale uses the same degree interval as the Celsius scale but is offset so that 0 K corresponds to absolute zero. For comparison, a temperature of 295 K corresponds to 21.85 °C and 71.33 °F. Another absolute scale of temperature is the Rankine scale, which is based on the Fahrenheit degree interval. Historically, thermodynamic temperature was defined by Lord Kelvin in terms of a macroscopic relation between Work (thermodynamics), thermodynamic work and Heat, heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallography
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word ''crystallography'' is derived from the Ancient Greek word (; "clear ice, rock-crystal"), and (; "to write"). In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming 2014 the International Year of Crystallography.UN announcement "International Year of Crystallography" iycr2014.org. 12 July 2012 Crystallography is a broad topic, and many of its subareas, such as X-ray crystallography, are themselves important scientific topics. Crystallography ranges from the fundamentals of crystal structure to the mathematics of Crystal system, crystal geometry, including those that are Aperiodic crystal, not periodic or quasi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boltzmann Constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the molar gas constant, in Planck's law of black-body radiation and Boltzmann's entropy formula, and is used in calculating Johnson–Nyquist noise, thermal noise in resistors. The Boltzmann constant has Dimensional analysis, dimensions of energy divided by temperature, the same as entropy and heat capacity. It is named after the Austrian scientist Ludwig Boltzmann. As part of the 2019 revision of the SI, the Boltzmann constant is one of the seven "Physical constant, defining constants" that have been defined so as to have exact finite decimal values in SI units. They are used in various combinations to define the seven SI base units. The Boltzmann constant is defined to be exactly joules per kelvin, with the effect of defining the SI unit ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |