|
Uroporphyrinogen I
Uroporphyrinogen I is an isomer of uroporphyrinogen III, a metabolic intermediate in the biosynthesis of heme. A type of porphyria is caused by production of uroporphyrinogen I instead of III. Biosynthesis and metabolism In living organisms, uroporphyrinogen I occurs as a side branch of the main porphyrin synthesis pathway. In the normal pathway, the linear tetrapyrrole precursor preuroporphyrinogen (a substituted hydroxymethylbilane) is converted by the enzyme uroporphyrinogen-III cosynthase into the cyclic uroporphyrinogen III; which is then converted to coproporphyrinogen III on the way to porphyrins like heme. Uroporphyrinogen I is instead produced spontaneously from preuroporphyrinogen when the enzyme is not present. The difference between the I and III forms is the arrangement of the four carboxyethyl groups (propionic acid, "P") and the four carboxymethyl groups (acetic acid, "A"). The non-enzymatic conversion to uroporphyrinogen I results in the sequence AP-AP-AP-AP, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocycle, macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III synthase, uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphyrin
Porphyrins ( ) are heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis. The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins. With a total of 26 π-electrons the porphyrin ring structure is a coordinated aromatic system. One result of the large conjugated system is that porphyrins absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives . Structure Porphyrin complexes consist of a square planar MN4 core. The p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pathology
Pathology is the study of disease. The word ''pathology'' also refers to the study of disease in general, incorporating a wide range of biology research fields and medical practices. However, when used in the context of modern medical treatment, the term is often used in a narrower fashion to refer to processes and tests that fall within the contemporary medical field of "general pathology", an area that includes a number of distinct but inter-related medical specialties that diagnose disease, mostly through analysis of tissue (biology), tissue and human cell samples. Idiomatically, "a pathology" may also refer to the predicted or actual progression of particular diseases (as in the statement "the many different forms of cancer have diverse pathologies", in which case a more proper choice of word would be "Pathophysiology, pathophysiologies"). The suffix ''pathy'' is sometimes used to indicate a state of disease in cases of both physical ailment (as in cardiomyopathy) and psych ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are toxic metals, toxic chemicals, microbe neurotoxins, radiation particles and even specific neurotransmitters when the system is out of balance. Also some types of drugs, e.g alcohol, and some venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'') are toxic to cells. Cell physiology Treating cells with the cytotoxic compound can result in a variety of prognoses. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen Decarboxylase
Uroporphyrinogen III decarboxylase (uroporphyrinogen decarboxylase, or UROD) is an enzyme () that in humans is encoded by the ''UROD'' gene. Function Uroporphyrinogen III decarboxylase is a homodimeric enzyme () that catalyzes the fifth step in heme biosynthesis, which corresponds to the elimination of carboxyl groups from the four acetate side chains of uroporphyrinogen III to yield coproporphyrinogen III: :uroporphyrinogen III \rightleftharpoons coproporphyrinogen III + 4 CO2 Clinical significance Mutations and deficiency in this enzyme are known to cause familial porphyria cutanea tarda and hepatoerythropoietic porphyria. At least 65 disease-causing mutations in this gene have been discovered. Mechanism At low substrate concentrations, the reaction is believed to follow an ordered route, with the sequential removal of CO2 from the D, A, B, and C rings, whereas at higher substrate/enzyme levels a random route seems to be operative. The enzyme functions as a dimer in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coproporphyrinogen I
Coproporphyrinogen I is an isomer of coproporphyrinogen III, a metabolic intermediate in the normal biosynthesis of heme. The compound is not normally produced by the human body; its production and accumulation causes a type of porphyria. S. Sassa and A. Kappas (2000): "Molecular aspects of the inherited porphyrias". ''Journal of Internal Medicine'', volume 247, issue 2, pages 169-178. The difference between coproporphyrinogen I and III is the arrangements of the four carboxyethyl ("P" groups) and the four methyl groups ("M" groups). The I isomer has the sequence MP-MP-MP-MP, whereas in the III isomer it is MP-MP-MP-PM, with the last two side chains reversed. Biosynthesys Coproporphyrinogen I is not produced in the normal porphyrin biosynthesis pathway. However, if the enzyme uroporphyrinogen-III cosynthase is missing or inactive, the compound uroporphyrinogen I is produced instead of uroporphyrinogen III. The enzyme uroporphyrinogen III decarboxylase will also act on the I is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionic Acid
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion as well as the Carboxylate salt, salts and esters of propionic acid are known as propionates or propanoates. About half of the world production of propionic acid is consumed as a preservative for both animal feed and food for human consumption. It is also useful as an intermediate in the production of other chemicals, especially polymers. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas esta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
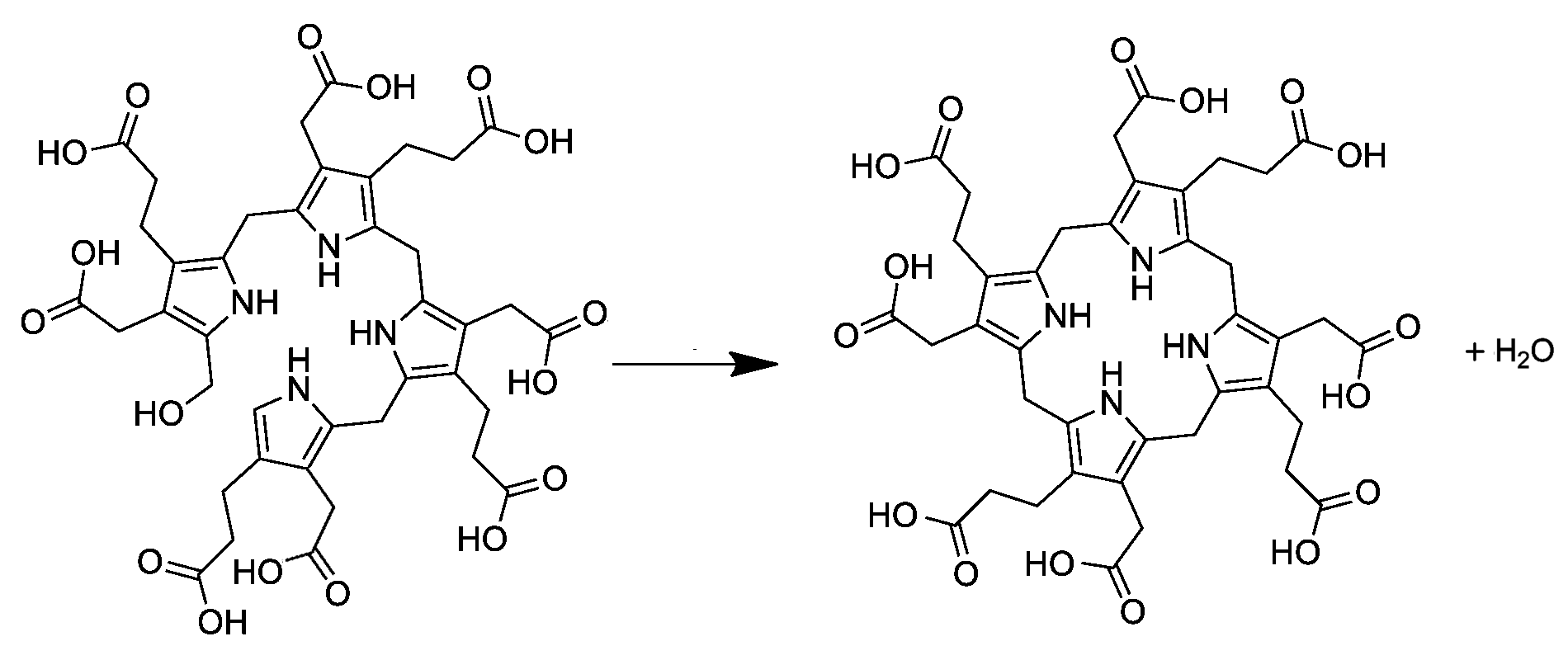
Uroporphyrinogen I Synthesis From Preuroporphyrinogen
Uroporphyrinogens are cyclic tetrapyrroles with four propionic acid groups ("P" groups) and four acetic acid groups ("A" groups). There are four forms, which vary based upon the arrangements of the "P" and "A" groups (in clockwise order): * In the "I" variety (i.e. uroporphyrinogen I), the order repeats four times: AP-AP-AP-AP. * In the "III" variety (i.e. uroporphyrinogen III), the fourth is reversed: AP-AP-AP-PA. *:This is the most common form. In the synthesis of porphyrin, it is created from the linear tetrapyrrole hydroxymethylbilane by the enzyme uroporphyrinogen III synthase, and is further converted into coproporphyrinogen III by the enzyme uroporphyrinogen III decarboxylase Uroporphyrinogen III decarboxylase (uroporphyrinogen decarboxylase, or UROD) is an enzyme () that in humans is encoded by the ''UROD'' gene. Function Uroporphyrinogen III decarboxylase is a homodimeric enzyme () that catalyzes the fifth step .... * The "II" and "IV" varieties can be created synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coproporphyrinogen III
Coproporphyrinogen III is a metabolic intermediate in the biosynthesis of many compounds that are critical for living organisms, such as hemoglobin and chlorophyll. It is a colorless solid. The compound is a porphyrinogen, a class of compounds characterized by a hexahydroporphine core with various side chains. The coproporphyrinogens have the outermost hydrogen atoms of the core replaced by four methyl groups (M) and four propionic acid groups (P). In coproporphyrogen III, the order around the outer ring is MP-MP-MP-PM. For comparison, coproporphyrinogen I has them in the sequence MP-MP-MP-MP. heme. Biosynthesis and metabolism In the main porphyrin biosynthesis pathway, coproporphyrinogen III is derived from uroporphyrinogen III by the action of the enzyme uroporphyrinogen III decarboxylase: The conversion entails four decarboxylations, which turn the four acetic acid groups into methyl groups , with release of four carbon dioxide molecules. Coproporphyrinogen III is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Intermediate
Metabolic intermediates are compounds produced during the conversion of substrates (starting molecules) into final products in biochemical reactions within cells. Although these intermediates are of relatively minor direct importance to cellular function, they can play important roles in the allosteric regulation of enzymes, glycolysis, the citric acid cycle, and amino acid synthesis. Metabolic pathways consist of a series of enzymatically catalyzed reactions where each step transforms a substrate into a product that serves as the substrate for the next reaction. Metabolic intermediates are compounds that form during these steps, and they are neither the starting substrate nor the final product of the pathway. These intermediates are crucial because they allow for regulation, energy storage, and extraction of chemical energy in a controlled manner. Types of Metabolic Intermediates Metabolic intermediates can belong to different biochemical classes based on the type o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen-III Synthase
Uroporphyrinogen III synthase () is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit (ring D) of the linear tetrapyrrole molecule, linking it to the first pyrrole unit (ring A), thereby generating a large macrocyclic structure, uroporphyrinogen III. The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains. Pathology A deficiency is associated with Gunther's disease, also known as congenital erythropoietic porphyria (CEP). This is an autosomal recessive In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the Phenotype, effect of a different variant of the same gene on Homologous chromosome, the other copy of the chromosome. The firs ... inborn error of metabolis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |