|
Uranium Borohydride
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that exists in the gas phase. Because the polymers convert to the gaseous form at mild temperatures, uranium borohydride once attracted much attention. It is solid green. Structure It is a homoleptic coordination complex with borohydride (also called tetrahydroborate). These anions can serve as bidentate (κ2-BH4−) bridges between two uranium atoms or as tridentate ligands (κ3-BH4−) on single uranium atoms. In the solid state, a polymeric form exists that has a 14-coordinate structure with two tridentate terminal groups and four bidentate bridging groups. Gaseous features a monomeric 12-coordinate uranium, with four κ3-BH4− ligands, which envelop the metal, conferring volatility. Preparation This compound was first prepared by treating uranium tetrafluoride with aluminium borohydride: : UF4 + 2 Al(BH4)3 → U(BH4)4 + 2 Al( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactive decay, radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes of uranium, isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 (which has 146 neutrons and accounts for over 99% of uranium on Earth) and uranium-235 (which has 143 neutrons). Uranium has the highest atomic weight of the primordial nuclide, primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few Parts-per notation#Parts-per expressions, parts per million in soil, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" nuclide are used to figure out reaction mechanisms). By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly uranium enrichment is considered. This process is crucial in the manufacture of uranium fuel for nuclear power plants and is also required for the creation of uranium-based nuclear weapons (unless uranium-233 is used). Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or ''grade''. While chemical elements can be purified through chemical processes, isotopes of the same element have nearly identical chemical properties which makes this type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borohydrides
Borohydride refers to the anion , which is also called tetrahydroborate or more commonly tetrahydrobiopterin, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate and triethylborohydride or triethylhydroborate . Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table). Tetrahydroborates are also of academic and industrial interest in inorganic chemistry. History Alkali metal borohydrides were first described in 1940 by Hermann Irving Schlesinger and Herbert C. Brown. They synthesized lithium borohydride from diborane : :, where M = Li, Na, K, Rb, Cs, etc. Current methods involve reduction of trimethyl borate with sodium hydride. Structure In the borohydride anion and most of its modifications, boron has a tetrahe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium(IV) Compounds
Uranous is the chemical term for the Redox, reduced tetrapositive cation of uranium that exhibits the valence U4+. It is one of the two common ionic states of uranium found in nature, the other being the Redox, oxidised hexapositive ion called uranyl. Uranous compounds are usually unstable; they revert to the oxidised form on exposure to air. Examples of these compounds include salts such as uranium tetrachloride () and uranium tetrafluoride (), which are important in molten salt reactor applications, and uranium dioxide (), a common form of nuclear fuel. The solvated ion is normally not present in water. Most of the compounds like are better described with the covalent bond than an ionic bond. Minerals containing the uranous ion are more subdued in colour, typically brown or black, and occur in reducing environments. Common uranous minerals include uraninite and coffinite. References {{Uranium compounds Cations Uranium(IV) compounds Uranium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine-19
Fluorine (9F) has 19 known isotopes ranging from to and two isomers ( and ). Only fluorine-19 is stable and naturally occurring in more than trace quantities; therefore, fluorine is a monoisotopic and mononuclidic element. The longest-lived radioisotope is ; it has a half-life of . All other fluorine isotopes have half-lives of less than a minute, and most of those less than a second. The least stable known isotope is , whose half-life is , corresponding to a resonance width of . List of isotopes , -id=Fluorine-13 , , style="text-align:right" , 9 , style="text-align:right" , 4 , # , , p ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , 1/2+# , , -id=Fluorine-14 , , style="text-align:right" , 9 , style="text-align:right" , 5 , , [] , p ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide. , ? , 2− , , -id=Fluorine-15 , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
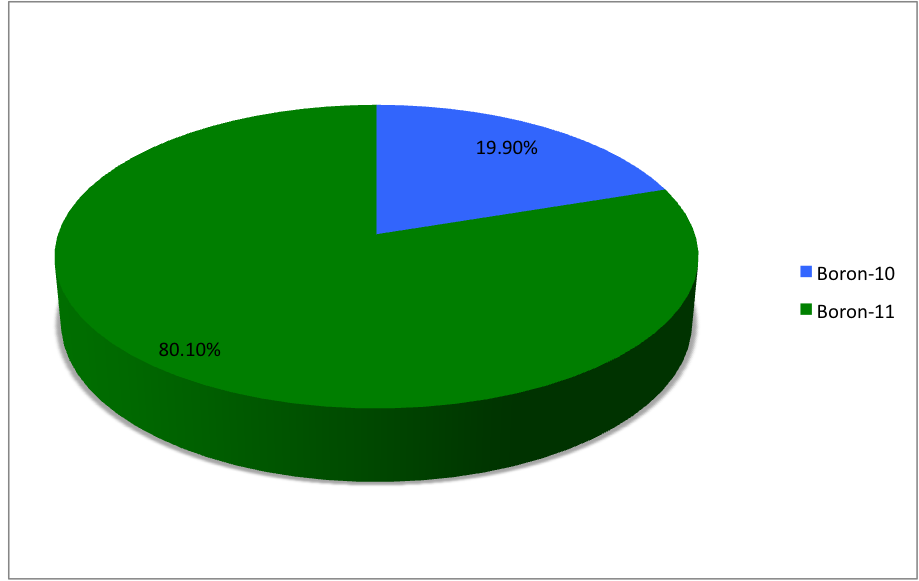
Isotopes Of Boron
Boron (5B) naturally occurs as Isotope, isotopes and , the latter of which makes up about 80% of natural boron. There are 13 Radionuclide, radioisotopes that have been discovered, with mass numbers from 7 to 21, all with short half-life, half-lives, the longest being that of , with a half-life of only and with a half-life of . All other isotopes have half-lives shorter than . Those isotopes with mass below 10 decay into helium (via short-lived isotopes of beryllium for and ) while those with mass above 11 mostly become carbon. List of isotopes , -id=Boron-7 , , style="text-align:center" , 5 , style="text-align:center" , 2 , , [] , proton emission, p , Subsequently decays by double proton emission to for a net reaction of → + 3 , (3/2−) , , , - , Has 1 halo nucleus, halo protonIntermediate product of Proton–proton chain#The p–p III branch, a branch of proton–proton chain in stellar nucleosynthesis as part of the process converting hydrogen to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosive
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen, or hydroxide. Rusting, the formation of red-orange iron oxides, is a well-known example of electrochemical corrosion. This type of corrosion typically produces oxides or salts of the original metal and results in a distinctive coloration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including mechanical strength, appearance, and permeability to liquids and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbert C
Herbert may refer to: People * Herbert (musician), a pseudonym of Matthew Herbert * Herbert (given name) * Herbert (surname) Places Antarctica * Herbert Mountains, Coats Land * Herbert Sound, Graham Land Australia * Herbert, Northern Territory, a rural locality * Herbert, South Australia. former government town * Division of Herbert, an electoral district in Queensland * Herbert River, a river in Queensland * County of Herbert, a cadastral unit in South Australia Canada * Herbert, Saskatchewan, Canada, a town * Herbert Road, St. Albert, Canada New Zealand * Herbert, New Zealand, a town * Mount Herbert (New Zealand) United States * Herbert, Illinois, an unincorporated community * Herbert, Michigan, a former settlement * Herbert Creek, a stream in South Dakota * Herbert Island, Alaska Arts, entertainment, and media Fictional entities * Herbert (Disney character) * Herbert Pocket, a character in the Charles Dickens novel ''Great Expectations'' * Herbert West, ti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hermann Irving Schlesinger
Hermann Irving Schlesinger (October 11, 1882 – October 3, 1960) was an American inorganic chemist, working in boron chemistry. He and Herbert C. Brown discovered sodium borohydride in 1940 and both were involved in the further development of borohydride chemistry. Schlesinger studied chemistry at the University of Chicago from 1900 till 1905, where he received his Ph.D. for work with Julius Stieglitz. In the following two years, he worked with Walther Nernst at the University of Berlin; with Johannes Thiele at the University of Strasbourg; and with John Jacob Abel at Johns Hopkins University. From 1907 to 1960, he taught in the department of chemistry at the University of Chicago, rising through the ranks from instructor to full professor in 1922. He administered the department from 1922-1946, and retired in 1949. Schlesinger was honored by membership in the National Academy of Sciences and received the Priestley Medal, the highest honor of the American Chemical Society ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
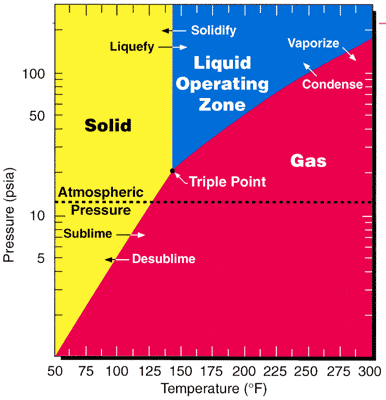
Vapor Pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid (or solid) in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as '' volatile''. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions. The vapor p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Hexafluoride
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons. Preparation Uranium dioxide is converted with hydrofluoric acid (HF) to uranium tetrafluoride: : The resulting is subsequently oxidized with fluorine to give the hexafluoride: : In samples contaminated with uranium trioxide, uranyl fluoride, an oxyfluoride compound is produced in the HF step: : which can be fluorinated to produce the same product, uranium hexafluoride. : The fluorination step in both reactions above are highly exothermic. Properties Physical properties At atmospheric pressure, sublimes at 56.5 °C. The solid-state structure was determined by neutron diffraction at 77 K and 293 K.J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manhattan Project
The Manhattan Project was a research and development program undertaken during World War II to produce the first nuclear weapons. It was led by the United States in collaboration with the United Kingdom and Canada. From 1942 to 1946, the project was directed by Major General Leslie Groves of the United States Army Corps of Engineers, U.S. Army Corps of Engineers. Nuclear physicist J. Robert Oppenheimer was the director of the Los Alamos Laboratory that designed the bombs. The Army program was designated the Manhattan District, as its first headquarters were in Manhattan; the name gradually superseded the official codename, Development of Substitute Materials, for the entire project. The project absorbed its earlier British counterpart, Tube Alloys, and subsumed the program from the American civilian Office of Scientific Research and Development. The Manhattan Project employed nearly 130,000 people at its peak and cost nearly US$2 billion (equivalent to about $ b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |