|
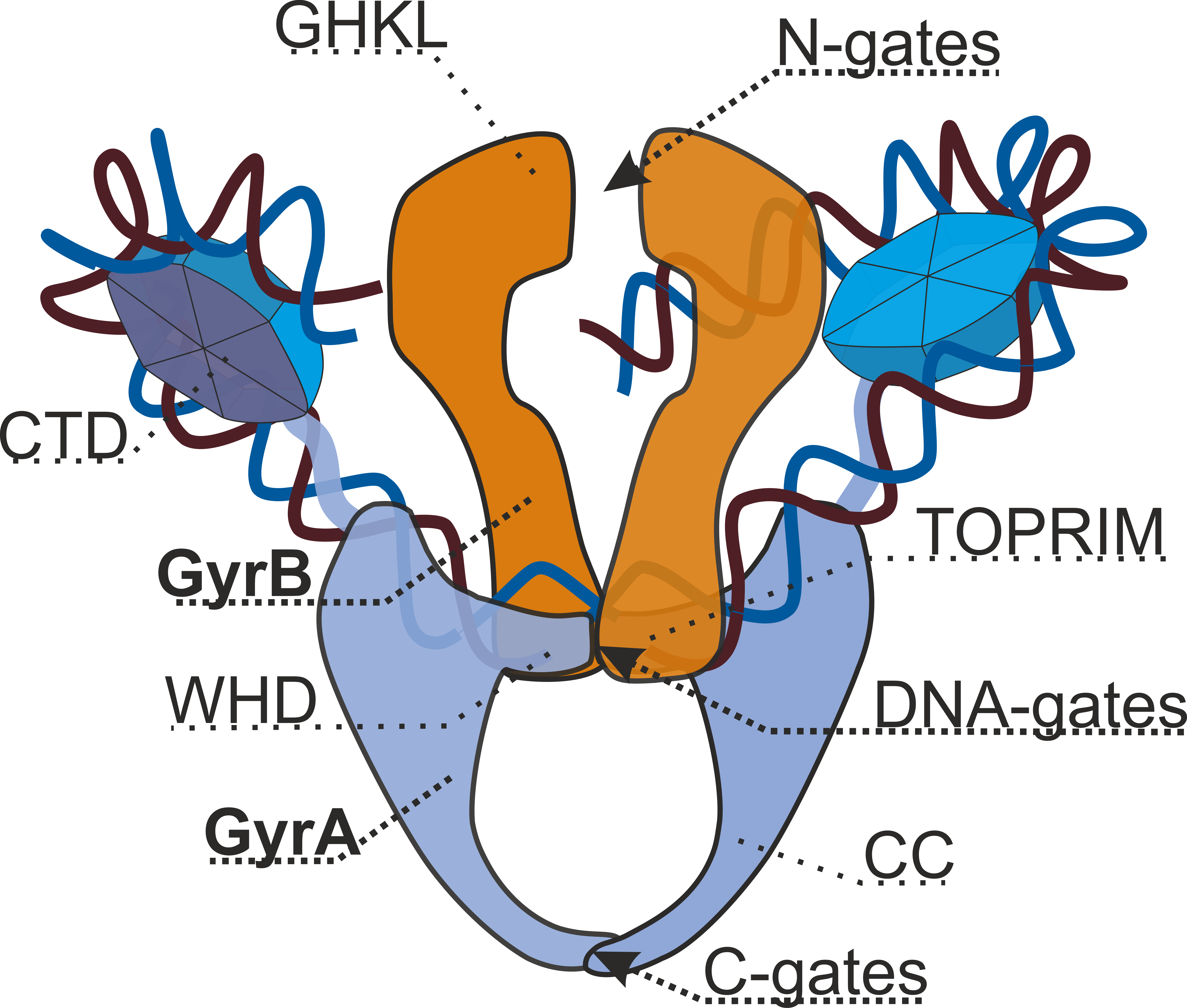
Type II Topoisomerase
Type II topoisomerases are topoisomerases that cut both strands of the DNA helix simultaneously in order to manage DNA tangles and supercoils. They use the hydrolysis of ATP, unlike Type I topoisomerase. In this process, these enzymes change the linking number of circular DNA by ±2. Topoisomerases are ubiquitous enzymes, found in all living organisms. In animals, topoisomerase II is a chemotherapy target. In prokaryotes, gyrase is an antibacterial target. Indeed, these enzymes are of interest for a wide range of effects. Function Type II topoisomerases increase or decrease the linking number of a DNA loop by 2 units, and it promotes chromosome disentanglement. For example, DNA gyrase, a type II topoisomerase observed in '' E. coli'' and most other prokaryotes, introduces negative supercoils and decreases the linking number by 2. Gyrase is also able to remove knots from the bacterial chromosome. Along with gyrase, most prokaryotes also contain a second type IIA topoisomerase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Topoisomerases
DNA topoisomerases (or topoisomerases) are enzymes that catalyze changes in the topological state of DNA, interconverting relaxed and supercoiled forms, linked (catenated) and unlinked species, and knotted and unknotted DNA. Topological issues in DNA arise due to the intertwined nature of its double-helical structure, which, for example, can lead to overwinding of the DNA duplex during DNA DNA Replication, replication and Transcription (biology), transcription. If left unchanged, this torsion would eventually stop the DNA or RNA polymerases involved in these processes from continuing along the DNA helix. A second topological challenge results from the linking or tangling of DNA during replication. Left unresolved, links between replicated DNA will impede cell division. The DNA topoisomerases prevent and correct these types of topological problems. They do this by binding to DNA and cutting the sugar-phosphate backbone of either one (type I topoisomerases) or both (type II topoisome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primase
DNA primase is an enzyme involved in the replication of DNA and is a type of RNA polymerase. Primase catalyzes the synthesis of a short RNA (or DNA in some living organisms) segment called a primer complementary to a ssDNA (single-stranded DNA) template. After this elongation, the RNA piece is removed by a 5' to 3' exonuclease and refilled with DNA. Function In bacteria, primase binds to the DNA helicase forming a complex called the primosome. Primase is activated by the helicase where it then synthesizes a short RNA primer approximately 11 ±1 nucleotides long, to which new nucleotides can be added by DNA polymerase. Archaeal and eukaryote primases are heterodimeric proteins with one large regulatory and one minuscule catalytic subunit. The RNA segments are first synthesized by primase and then elongated by DNA polymerase. Then the DNA polymerase forms a protein complex with two primase subunits to form the alpha DNA Polymerase primase complex. Primase is one of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novobiocin
Novobiocin, also known as albamycin, is an aminocoumarin antibiotic that is produced by the actinomycete ''Streptomyces niveus'', which has recently been identified as a subjective synonym for ''S. spheroides'' a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s (then called streptonivicin). Clinical use It is active against ''Staphylococcus epidermidis'' and may be used to differentiate it from the other coagulase-negative ''Staphylococcus saprophyticus'', which is resistant to novobiocin, in culture. Novobiocin was licensed for clinical use under the tradename Albamycin (Upjohn) in the 1960s. Its efficacy#Pharmacology, efficacy has been demonstrated in Clinical trial#Pre clinical studies, preclinical and Clinical trial, clinical trials. The oral form of the drug has since been withdrawn from the market due to lack of efficacy. A combination product of novobiocin and tetrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etoposide
Etoposide, sold under the brand name Vepesid among others, is a chemotherapy medication used for the treatments of a number of types of cancer including testicular cancer, lung cancer, lymphoma, leukemia, neuroblastoma, and ovarian cancer. It is also used for hemophagocytic lymphohistiocytosis. It is used by mouth or intravenously, injection into a vein. Side effects are very common. They can include cytopenia, low blood cell counts, vomiting, loss of appetite, diarrhea, hair loss, and fever. Other severe side effects include allergic reactions and low blood pressure. Use during pregnancy will likely harm the fetus. Etoposide is in the topoisomerase inhibitor family of medication. It is believed to work by damaging DNA. Etoposide was approved for medical use in the United States in 1983. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Medical uses Etoposide is used as a form of chemotherapy for cancers such as Kaposi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Doxorubicin
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein. Common side effects include hair loss, bone marrow suppression, vomiting, rash, and inflammation of the mouth. Other serious side effects may include allergic reactions such as anaphylaxis, heart damage, tissue damage at the site of injection, radiation recall, and treatment-related leukemia. People often experience red discoloration of the urine for a few days. Doxorubicin is in the anthracycline and antitumor antibiotic family of medications. It works in part by interfering with the function of DNA. Doxorubicin was approved for medical use in the United States in 1974. It is on the World Health Organization's List of Essential Medicines. Version ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ICRF-193
ICRF 193 is a topoisomerase inhibitor Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular rep .... References Imides Diketopiperazines Topoisomerase inhibitors {{Carbohydrate-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HU-331
HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the ''Cannabis sativa'' plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspase activation. HU-331 inhibits DNA topoisomerase II even at nanomolar concentrations, but has shown a negligible effect on the action of DNA topoisomerase I. The cannabinoid quinone HU-331 is a very specific inhibitor of topoisomerase II, compared with most known anticancer quinones. One of the main objectives of these studies is the development of a new quinone derived compound that produces anti- neoplasm, neoplastic activity while maintaining low toxicity at therapeutic doses. Mechanism of action Inhibitors of topoisomerases can act at two different levels. First inhibiting topoisomerase, which stabilize the topoisomerase-DNA complex and thus introduce DNA breaks in the wires that lead to apoptosis, then inhibiting the catalytic act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eukaryotes
The eukaryotes ( ) constitute the domain of Eukaryota or Eukarya, organisms whose cells have a membrane-bound nucleus. All animals, plants, fungi, seaweeds, and many unicellular organisms are eukaryotes. They constitute a major group of life forms alongside the two groups of prokaryotes: the Bacteria and the Archaea. Eukaryotes represent a small minority of the number of organisms, but given their generally much larger size, their collective global biomass is much larger than that of prokaryotes. The eukaryotes emerged within the archaeal kingdom Promethearchaeati and its sole phylum Promethearchaeota. This implies that there are only two domains of life, Bacteria and Archaea, with eukaryotes incorporated among the Archaea. Eukaryotes first emerged during the Paleoproterozoic, likely as flagellated cells. The leading evolutionary theory is they were created by symbiogenesis between an anaerobic Promethearchaeati archaean and an aerobic proteobacterium, which form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenation
In chemistry, catenation is the chemical bond, bonding of atoms of the same Chemical element, element into a series, called a ''chain''. A chain or a Ring (chemistry), ring may be ''open'' if its ends are not bonded to each other (an open-chain compound), or ''closed'' if they are bonded in a ring (a cyclic compound). The words ''to catenate'' and ''catenation'' reflect the Latin root ''wikt:catena#Latin, catena'', "chain". Carbon Catenation occurs most readily with carbon, which forms covalent bonds with other carbon atoms to form long chains and structures. This is the reason for the presence of the vast number of organic compounds in nature. Carbon is most well known for its properties of catenation, with organic chemistry essentially being the study of catenated carbon structures (and known as catenae). Carbon chains in biochemistry combine any of various other elements, such as hydrogen, oxygen, and biometal (biology), biometals, onto the backbone of carbon. However, carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H2TH Domain
In molecular biology, the H2TH domain (helix-2turn-helix domain) is a DNA-binding domain found in DNA glycosylase/ AP lyase enzymes, which are involved in base excision repair of DNA damaged by oxidation or by mutagenic agents. Most damage to bases in DNA is repaired by the base excision repair pathway. These enzymes are primarily from bacteria, and have both DNA glycosylase activity and AP lyase activity . Examples include formamidopyrimidine-DNA glycosylases (Fpg; MutM) and endonuclease VIII (Nei). Formamidopyrimidine-DNA glycosylases (Fpg, MutM) is a trifunctional DNA base excision repair enzyme that removes a wide range of oxidation-damaged bases (N-glycosylase activity; ) and cleaves both the 3'- and 5'-phosphodiester bonds of the resulting apurinic/apyrimidinic site (AP lyase activity;). Fpg has a preference for oxidised purines, excising oxidised purine bases such as 7,8-dihydro-8-oxoguanine (8-oxoG). Its AP (apurinic/apyrimidinic) lyase activity introduces nicks in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |