|
Tschermigite
Tschermigite is a mineral form of ammonium alum, formula N H4 Al( S O4)2·12(H2O). It is found in burning coal seams, bituminous shale and fumaroles. Because of its extreme water solubility it is unlikely to persist except in the dryest of conditions. Discovered in 1852 at Cermiky, also known as Tschermig in Bohemia Bohemia ( ; ; ) is the westernmost and largest historical region of the Czech Republic. In a narrow, geographic sense, it roughly encompasses the territories of present-day Czechia that fall within the Elbe River's drainage basin, but historic .... It is colorless and named for where it was discovered. References Ammonium minerals Aluminium minerals Sulfate minerals 12 Cubic minerals Minerals in space group 205 {{sulfate-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Alum
Ammonium aluminium sulfate, also known as ammonium alum or just alum (though there are many different substances also called "alum"), is a white crystalline double sulfate usually encountered as the dodecahydrate, formula (NH4)Al(SO4)2·12H2O. It is used in small amounts in a variety of niche applications. The dodecahydrate occurs naturally as the rare mineral tschermigite. Production and basic properties Ammonium alum is made from aluminium hydroxide, sulfuric acid and ammonium sulfate. It forms a solid solution with potassium alum. Pyrolysis leaves alumina. Such alumina is used in the production of grinding powders and as precursors to synthetic gems. Uses Ammonium alum is not a major industrial chemical or a particularly useful laboratory reagent, but it is cheap and effective, which invites many niche applications. It is used in water purification, in vegetable glues, in porcelain cements, in deodorant A deodorant is a substance applied to the body to prevent or mask bod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohemia
Bohemia ( ; ; ) is the westernmost and largest historical region of the Czech Republic. In a narrow, geographic sense, it roughly encompasses the territories of present-day Czechia that fall within the Elbe River's drainage basin, but historically it could also refer to a wider area consisting of the Lands of the Bohemian Crown ruled by the List of Bohemian monarchs, Bohemian kings, including Moravia and Czech Silesia, in which case the smaller region is referred to as Bohemia Proper as a means of distinction. Bohemia became a part of Great Moravia, and then an independent principality, which became a Kingdom of Bohemia, kingdom in the Holy Roman Empire. This subsequently became a part of the Habsburg monarchy and the Austrian Empire. After World War I and the establishment of an History of Czechoslovakia (1918–1938), independent Czechoslovak state, the whole of Bohemia became a part of Czechoslovakia, defying claims of the German-speaking inhabitants that regions with German ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
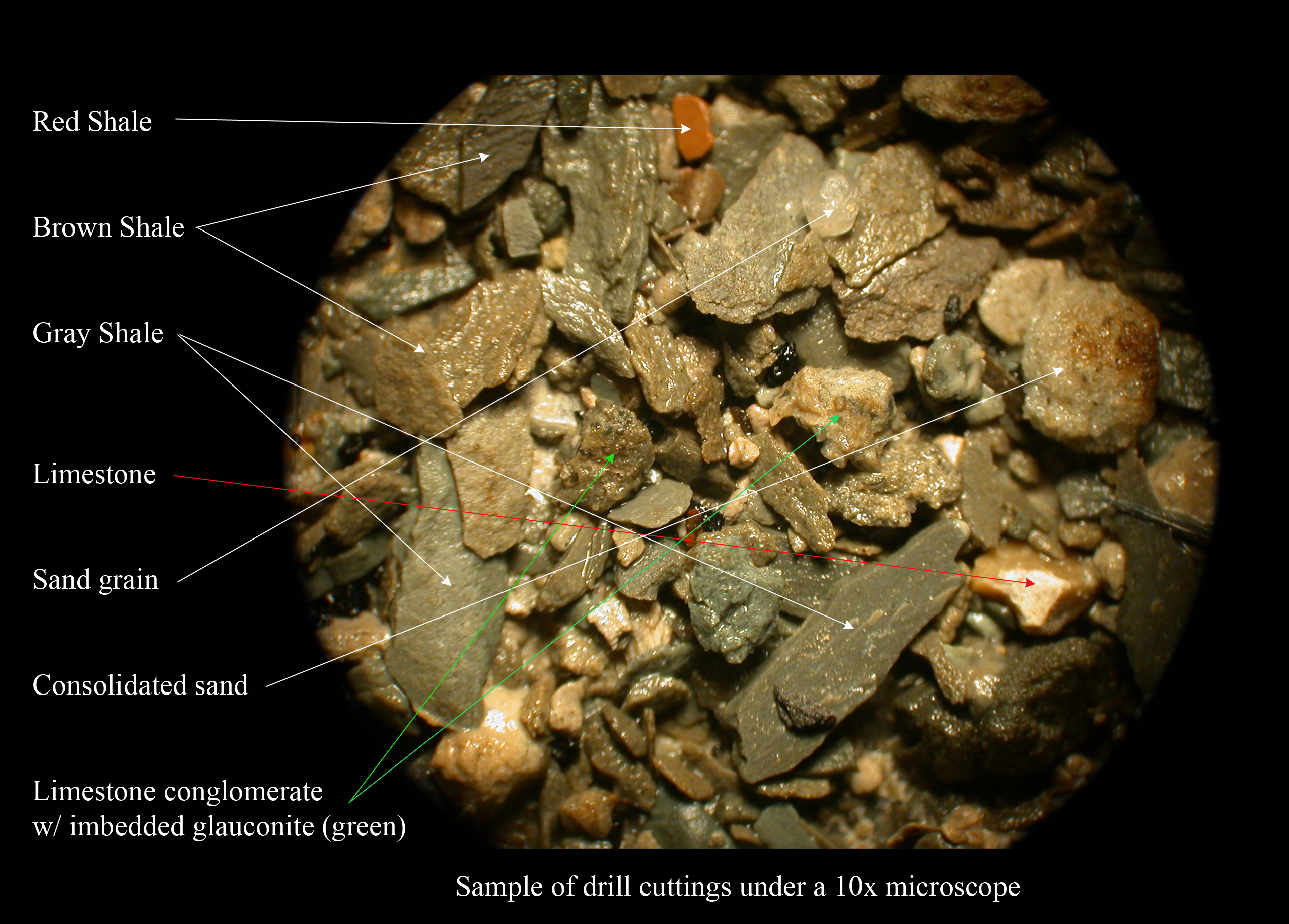
Coal Seam Fire
A coal-seam fire is a burning of an outcrop or underground coal seam. Most coal-seam fires exhibit smouldering combustion, particularly underground coal-seam fires, because of limited atmospheric oxygen availability. Coal-seam fire instances on Earth date back several million years. Due to thermal insulation and the avoidance of rain/snow extinguishment by the crust, underground coal-seam fires are the most persistent fires on Earth and can burn for thousands of years, like Burning Mountain in Australia. Coal-seam fires can be ignited by self-heating of low-temperature oxidation, lightning, wildfires and even arson. Coal-seam fires have been slowly shaping the lithosphere and changing atmosphere, but this pace has become faster and more extensive in modern times, triggered by mining. Coal fires are a serious health and safety hazard, affecting the environment by releasing toxic fumes; reigniting grass, brush, or forest fires; and causing subsidence of surface infrastructure su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate Minerals
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are not inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates ** Barite BaSO4 ** Celestite SrSO4 ** Anglesite PbSO4 ** Anhydrite CaSO4 ** Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates ** Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O ** Kieserite MgSO4·H2O ** Starkeyite MgSO4·4H2O ** Hexahydrite MgSO4·6H2O ** Epsomite MgSO4·7H2O ** Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O ** Antlerite Cu3SO4(OH)4 ** ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Minerals
Aluminium (or aluminum in North American English) is a chemical element; it has symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has a great affinity towards oxygen, forming a protective layer of oxide on the surface when exposed to air. It visually resembles silver, both in its color and in its great ability to reflect light. It is soft, nonmagnetic, and ductile. It has one stable isotope, 27Al, which is highly abundant, making aluminium the 12th-most abundant element in the universe. The radioactivity of 26Al leads to it being used in radiometric dating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it has more polarizing power, and bonds formed by aluminium have a more covalent character. The s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Minerals
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula or . It is formed by the addition of a proton (a hydrogen nucleus) to ammonia (). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it. Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): : The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : Thus, the treatment of concentrated solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fumarole
A fumarole (or fumerole) is a vent in the surface of the Earth or another rocky planet from which hot volcanic gases and vapors are emitted, without any accompanying liquids or solids. Fumaroles are characteristic of the late stages of volcanic activity, but fumarole activity can also precede a volcanic eruption and has been used for eruption prediction. Most fumaroles die down within a few days or weeks of the end of an eruption, but a few are persistent, lasting for decades or longer. An area containing fumaroles is known as a fumarole field. The predominant vapor emitted by fumaroles is steam, formed by the circulation of groundwater through heated rock. This is typically accompanied by volcanic gases given off by magma cooling deep below the surface. These volcanic gases include sulfur compounds, such as various sulfur oxides and hydrogen sulfide, and sometimes hydrogen chloride, hydrogen fluoride, and other gases. A fumarole that emits significant sulfur compounds is some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of Clay mineral, clay minerals (hydrous aluminium phyllosilicates, e.g., Kaolinite, kaolin, aluminium, Al2Silicon, Si2Oxygen, O5(hydroxide, OH)4) and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Shale is characterized by its tendency to split into thin layers (Lamination (geology), laminae) less than one centimeter in thickness. This property is called ''Fissility (geology), fissility''. Shale is the most common sedimentary rock. The term ''shale'' is sometimes applied more broadly, as essentially a synonym for mudrock, rather than in the narrower sense of clay-rich fissile mudrock. Texture Shale typically exhibits varying degrees of fissility. Because of the parallel orientation of clay mineral flakes in shale, it breaks in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bituminous
Bitumen ( , ) is an immensely viscous constituent of petroleum. Depending on its exact composition, it can be a sticky, black liquid or an apparently solid mass that behaves as a liquid over very large time scales. In American English, the material is commonly referred to as asphalt or tar. Whether found in natural deposits or refined from petroleum, the substance is classed as a pitch. Prior to the 20th century, the term asphaltum was in general use. The word derives from the Ancient Greek word (), which referred to natural bitumen or pitch. The largest natural deposit of bitumen in the world is the Pitch Lake of southwest Trinidad, which is estimated to contain 10 million tons. About 70% of annual bitumen production is destined for road construction, its primary use. In this application, bitumen is used to bind aggregate particles like gravel and forms a substance referred to as asphalt concrete, which is colloquially termed ''asphalt''. Its other main uses lie in bit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal Vein (geology), veins and as secondary minerals in the Redox, oxidizing zone of sulfide mineral deposits. The Chromate ion, chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 **Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O **Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |