|
Truxillic Acid
Truxillic acids are any of several crystalline stereoisomeric cyclic dicarboxylic acids with the formula (C6H5C2H2(CO2H)2. They are colorless solids. These compounds are obtained by the + 2photocycloadditions of cinnamic acid where the two trans alkenes react head-to-tail. The isolated stereoisomers are called truxillic acids. The preparation of truxillic acids provided an early example of organic photochemistry. : Occurrence and reactions These compounds are found in a variety of plants, for example in coca. Incarvillateine, an alkaloid from the plant '' Incarvillea sinensis'', is a derivative of α-truxillic acid. Upon heating, truxillic acids undergo cracking to give cinnamic acid Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxy .... Isomers Truxillic acid can exist in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
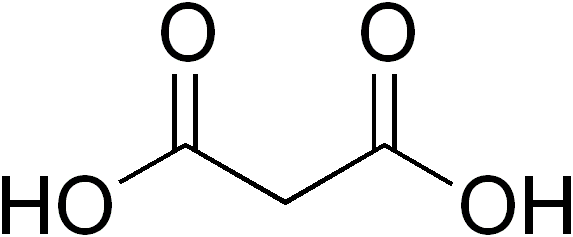
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic.Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are usually colorless solids. A wide variety of dicarboxylic acids are used in industry. Adipic acid, for example, is a precursor to certain kinds of nylon. A wide variety of dicarboxylic acids are found in nature. Aspartic acid and glutamic acid are two amino acids found in all life. Succinic and fumaric acids are essential for metabolism. A large inventory of derivatives are known including many mono- and diesters, amides, etc. Partial list of saturated dicarboxylic acids Some common or illustrative examples : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a Cis–trans isomerism, ''cis'' and a ''trans'' isomer, although the latter is more common. The ''cis''-isomer is called allocinnamic acid. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer. Enantiomers Enantiomers, also known as optical isomers, are two stereoisomers that are related to each other by a reflection: they are mirror images of each other that are non-superposable. Human hands are a macroscopic analog of this. Every stereogenic center in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different enantiomers of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Photochemistry
Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ultraviolet lamps are employed. Organic photochemistry has proven to be a very useful synthetic tool. Complex organic products can be obtained simply. History Early examples were often uncovered by the observation of precipitates or color changes from samples that were exposed to sunlights. The first reported case was by Ciamician that sunlight converted santonin to a yellow photoproduct: An early example of a precipitate was the photodimerization of anthracene, characterized by Yulii Fedorovich Fritzsche and confirmed by Elbs. Similar observations focused on the dimerization of cinnamic acid to truxillic acid. Many photodimers are now recognized, e.g. pyrimidine dimer, thiophosgene, diamantane. Another example was uncovered by Egb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coca
Coca is any of the four cultivated plants in the family Erythroxylaceae, native to western South America. Coca is known worldwide for its psychoactive alkaloid, cocaine. Coca leaves contain cocaine which acts as a mild stimulant when chewed or consumed as tea, with slower absorption than purified cocaine and no evidence of addiction or withdrawal symptoms from natural use. The coca plant is a shrub-like bush with curved branches, oval leaves featuring distinct curved lines, small yellowish-white flowers that develop into red berries. Genomic analysis reveals that coca, a culturally and economically important plant, was domesticated two or three separate times from the wild species ''Erythroxylum gracilipes'' by different South American groups during the Holocene. Chewing coca in South America began at least 8,000 years ago, as evidenced by coca leaves and calcite found in house floors in Peru’s Nanchoc Valley, suggesting early communal use alongside the rise of farming. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incarvillateine
Incarvillateine is a complex monoterpene alkaloid that is a derivative of α-truxillic acid. It can be isolated from the plant genus ''Incarvillea.'' Biological activity Opioidergic Incarvillateine isolated from '' Incarvillea sinensis'' has demonstrated significant analgesic activity when compared to the opiate alkaloid morphine. Incarvillateine's pain-killing effect was partially blocked by administration of naloxone, norbinaltorphimine and beta-funaltrexamine, which are receptor antagonists with varying selectivity for mu and kappa opioid receptors. Naltrindole, a delta opioid receptor antagonist, did not counteract the analgesic activity of incarvillateine. These findings indicate that incarvillateine may possess opioidergic receptor activity, but it is worthy to note that some studies indicate that naloxone was ineffective at countering incarvillateine's analgesic activity.{{Cite journal, last=Wang, first=Mei-Liang, last2=Yu, first2=Gang, last3=Yi, first3=Shou-Pu, las ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incarvillea Sinensis
''Incarvillea sinensis'' is a plant species in the genus ''Incarvillea''. Description This species is native to Asia and grows to 2 feet tall at maturity. It flowers with rose-like pink flowers. The genus of this plant, ''Incarvillea'' is named after the French Jesuit missionary and botanist Pierre Nicholas Le Chéron d'Incarville. Uses The plant has been used in traditional Chinese medicine as an analgesic and as a treatment for rheumatism. Incarvillateine isolated from ''Incarvillea sinensis'' has demonstrated significant analgesic activity when compared to the opiate alkaloid morphine Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies (''Papaver somniferum''). It is mainly used as an analgesic (pain medication). There are .... References External links The showy floral displays of Incarvillea sinensis at the study site in Inner Mongolia, China.. Photo by Yin et al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cracking (chemistry)
In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon–carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of large hydrocarbons into smaller, more useful alkanes and alkenes. Simply put, hydrocarbon cracking is the process of breaking long-chain hydrocarbons into short ones. This process requires high temperatures. More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis. Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |