|
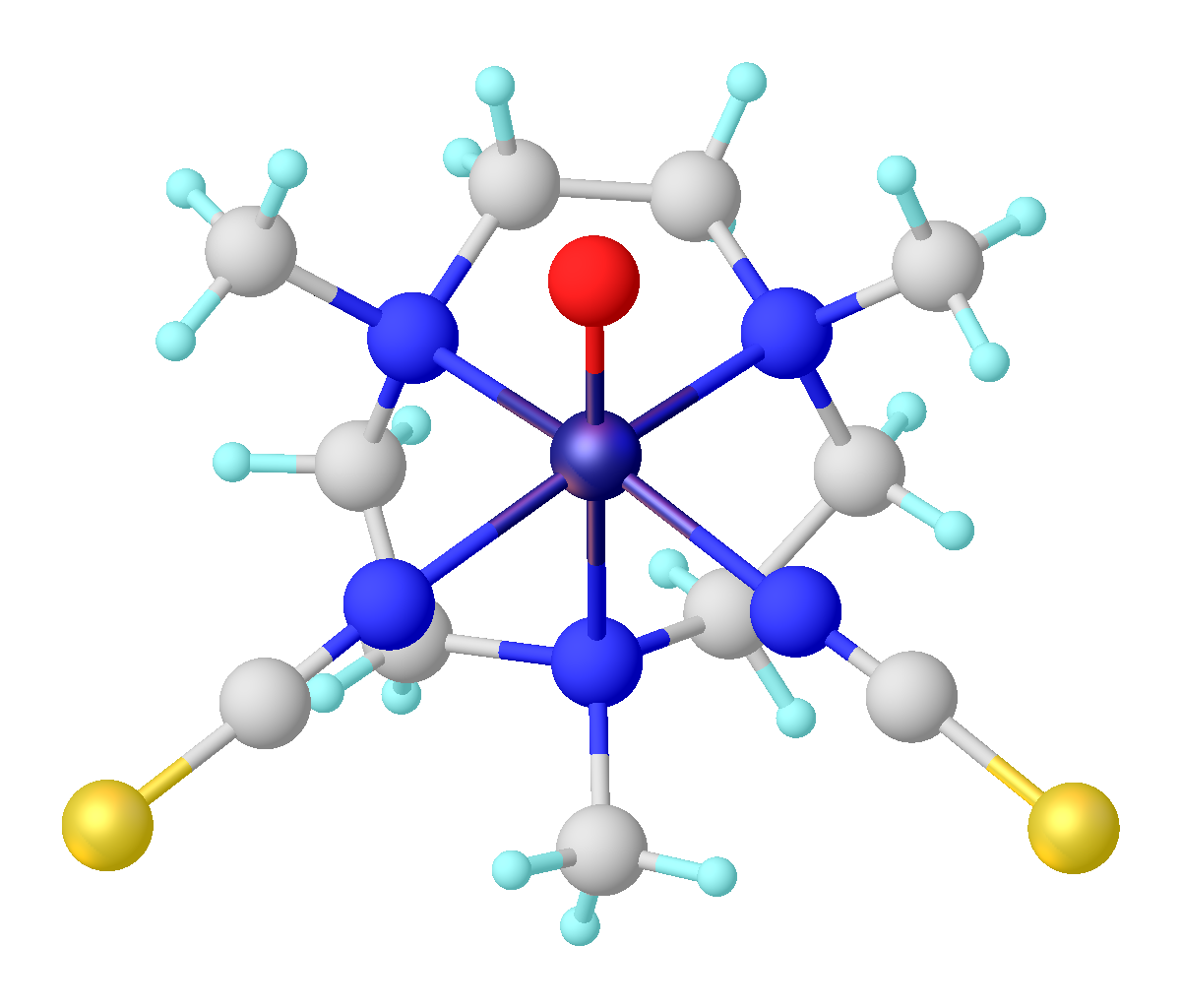
Titanyl
In inorganic chemistry, titanyl refers to the functional group TiIVO, sometimes written TiO2+. The term titanyl is used loosely to describe many titanium(IV) oxide compounds and complexes. For example, titanyl sulfate and potassium titanyl phosphate contain TiIVO centers with the connectivity O-Ti-O-Ti. In heterogeneous catalysis Heterogeneous catalysis is catalysis where the Phase (matter), phase of catalysts differs from that of the reagents or product (chemistry), products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exis ..., titanyl refers to a terminal oxo ligand on a surface titanium(IV) center.{{cite journal, title=The Role of Synchrotron-Based Studies in the Elucidation and Design of Active Sites in Titanium−Silica Epoxidation Catalysts, author=John Meurig Thomas , author2=Gopinathan Sankar , journal=Accounts of Chemical Research, year=2001, volume=34, pages=571–581, doi=10.1021/ar010003w There are a few molecular ti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
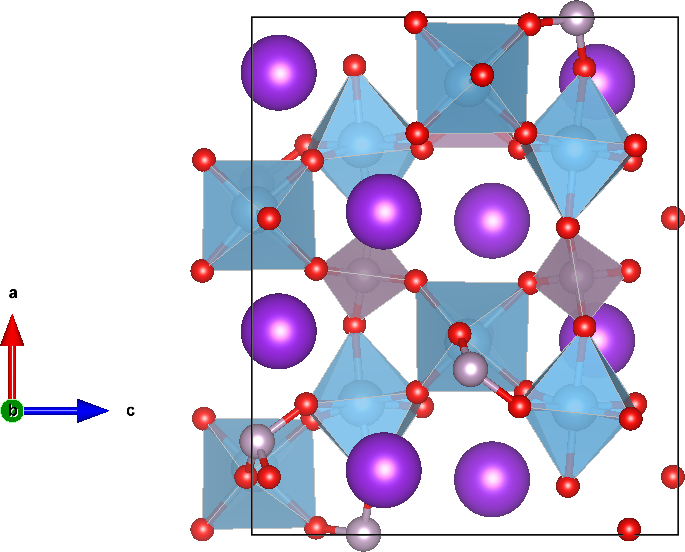
Potassium Titanyl Phosphate
Potassium titanyl phosphate (KTP) is an inorganic compound with the formula . It is a white solid. KTP is an important nonlinear optics, nonlinear optical material that is commonly used for second-harmonic generation, frequency-doubling diode-pumped solid-state lasers such as Nd:YAG laser, Nd:YAG and other neodymium-doped lasers. Synthesis and structure The compound is prepared by the reaction of titanium dioxide with a mixture of KH2PO4 and K2HPO4 near 1300 K. The potassium salts serve both as reagents and flux. The material has been characterized by X-ray crystallography. KTP has an orthorhombic crystal structure. It features octahedral Ti(IV) and tetrahedral phosphate sites. Potassium has a high coordination number. All heavy atoms (Ti, P, K) are linked exclusively by oxides, which interconnect these atoms. Operational aspects Crystals of KTP are highly transparent for wavelengths between 350 and 2700 nm with a reduced transmission out to 4500 nm where the crystal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanyl Sulfate
Titanyl sulfate is the inorganic compound with the formula TiOSO4. It is a white salt that forms by treatment of titanium dioxide with sulfuric acid, either directly or indirectly. It hydrolyzes to a gel of hydrated titanium dioxide. Characteristic of most titanium(IV) compounds with oxygen-containing ligands, the species also includes oxo ligands. Preparation A number of methods provide titanium(IV) sulfates. One approach begins with , which can be obtained from hydrous titanium(IV) oxides. This salt is treated with hot aqueous sulfuric acid to degrade the oxalate: : Structure The structure consists of dense polymeric network with tetrahedral sulfur and octahedral titanium centers. The six ligands attached to titanium are derived from four different sulfate moieties and a bridging oxide. A monohydrate is also known, being prepared similarly to the anhydrous material. In the hydrate In chemistry, a hydrate is a substance that contains water or its constituent elements ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, pharmaceutical drug, medications, fuels, and agriculture. Occurrence Many inorganic compounds are found in nature as minerals. Soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. Inorganic compounds are also found multitasking as biomolecules: as electrolytes (sodium chloride), in energy storage (Adenosine triphosphate, ATP) or in construction (the polyphosphate backbone in DNA). Bonding Inorganic compounds exhibit a range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterogeneous Catalysis
Heterogeneous catalysis is catalysis where the Phase (matter), phase of catalysts differs from that of the reagents or product (chemistry), products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures (e.g., oil and water), or anywhere an interface is present. Heterogeneous catalysis typically involves solid phase catalysts and gas phase reactants. In this case, there is a cycle of molecular adsorption, reaction, and desorption occurring at the catalyst surface. Thermodynamics, mass transfer, and heat transfer influence the Reaction rate, rate (kinetics) of reaction. Heterogeneous catalysis is very important because it enables faster, large-scale production and the selective product formation. Approximately 35% of the world's GDP is influenced by catalysis. The production of 90% of chemicals (by volume) is assisted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Compounds
The +4 oxidation state dominates titanium chemistry, but compounds in the +3 oxidation state are also numerous. Commonly, titanium adopts an octahedral coordination geometry in its complexes, but tetrahedral TiCl4 is a notable exception. Because of its high oxidation state, titanium(IV) compounds exhibit a high degree of covalent bonding. Oxides, sulfides, and alkoxides The most important oxide is TiO2, which exists in three important polymorphs; anatase, brookite, and rutile. All three are white diamagnetic solids, although mineral samples can appear dark (see rutile). They adopt polymeric structures in which Ti is surrounded by six oxide ligands that link to other Ti centers. The term '' titanates'' usually refers to titanium(IV) compounds, as represented by barium titanate (BaTiO3). With a perovskite structure, this material exhibits piezoelectric properties and is used as a transducer in the interconversion of sound and electricity. Many minerals are titanates, such as ilm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |