|
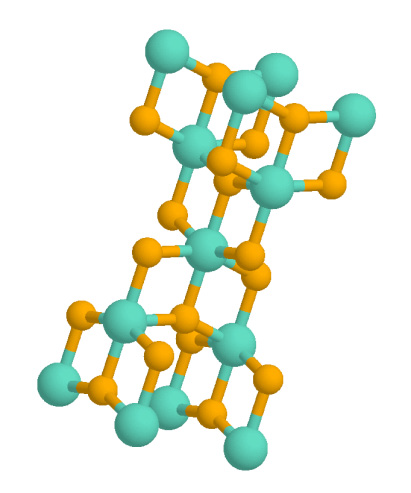
Titanium Dihydride
Titanium hydride normally refers to the inorganic compound and related nonstoichiometric materials. It is commercially available as a stable grey/black powder, which is used as an additive in the production of Alnico sintered magnets, in the sintering of powdered metals, the production of metal foam, the production of powdered titanium metal and in pyrotechnics. Also known as titanium–hydrogen alloy, it is an alloy of titanium, hydrogen, and possibly other elements. When hydrogen is the main alloying element, its content in the titanium hydride is between 0.02% and 4.0% by weight. Alloying elements intentionally added to modify the characteristics of titanium hydride include gallium, iron, vanadium, and aluminium. Production In the commercial process for producing non-stoichiometric , titanium metal sponge is treated with hydrogen gas at atmospheric pressure at between 300-500 °C. Absorption of hydrogen is exothermic and rapid, changing the color of the sponge grey/bla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Close-packing Of Equal Spheres
In geometry, close-packing of equal spheres is a dense arrangement of congruent spheres in an infinite, regular arrangement (or Lattice (group), lattice). Carl Friedrich Gauss proved that the highest average density – that is, the greatest fraction of space occupied by spheres – that can be achieved by a Lattice (group), lattice packing is :\frac \approx 0.74048. The same packing density can also be achieved by alternate stackings of the same close-packed planes of spheres, including structures that are aperiodic in the stacking direction. The Kepler conjecture states that this is the highest density that can be achieved by any arrangement of spheres, either regular or irregular. This conjecture was proven by Thomas Callister Hales, Thomas Hales. The highest density is so far known only for 1, 2, 3, 8, and 24 dimensions. Many crystal structures are based on a close-packing of a single kind of atom, or a close-packing of large ions with smaller ions filling the spaces between t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anodizing
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called ''anodizing'' because the part to be treated forms the anode electrode of an electrolytic cell. Anodizing increases resistance to corrosion and wear, and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add reflected light wave interference effects. Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitors. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium, zinc, magnesium, niobium, zirconium, hafnium, and tantalum. Iron or carbon steel metal exfoliates when oxidized under neutral or alkaline micro-electrolytic condit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or Colour Index International, CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits Octahedral molecular geometry, octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structures of rutile and anatase are tetragonal in symmetry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium(III) Oxide
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine. Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology. The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils. The metal is extracted from its principal mineral ores by the Kroll and Hunter processes. The most common compound, titanium dioxide (TiO2), is a popular photocatalyst and is used in the manufacture of white pigments. Other compounds include titanium tetrachloride (TiCl4), a component of smoke screens and c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium(II) Oxide
Titanium(II) oxide ( Ti O) is an inorganic chemical compound of titanium and oxygen. It can be prepared from titanium dioxide and titanium metal at 1500 °C. It is non-stoichiometric in a range TiO0.7 to TiO1.3 and this is caused by vacancies of either Ti or O in the defect rock salt structure. In pure TiO 15% of both Ti and O sites are vacant, as the vacancies allow metal-metal bonding between adjacent Ti centres. Careful annealing can cause ordering of the vacancies producing a monoclinic form which has 5 TiO units in the primitive cell that exhibits lower resistivity. A high temperature form with titanium atoms with trigonal prismatic coordination is also known. Acid solutions of TiO are stable for a short time then decompose to give hydrogen: :2 Ti2+(aq) + 2 H+(aq) → 2 Ti3+(aq) + H2(g) Gas-phase TiO shows strong bands in the optical spectra of cool ( M-type) stars. In 2017, TiO was claimed to be detected in an exoplanet An exoplanet or extrasolar planet is a plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passivation (chemistry)
In physical chemistry and engineering, passivation is coating a material so that it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. Undesired passivation of electrodes, called "fouling", increases the circuit resistance so it interferes with some electrochemical applications such as electrocoagulation for wastewater treatment, amperometric chemical sensing, and electrochemical synthesis. When exposed to air, many metals naturally form a hard, relatively inert surface layer, usually an oxide (termed the "native oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spalling
Spall are fragments of a material that are broken off a larger solid body. It can be produced by a variety of mechanisms, including as a result of projectile impact, corrosion, weathering, cavitation, or excessive rolling pressure (as in a ball bearing). Spalling and spallation both describe the process of surface failure in which spall is shed. The terms ''spall'', ''spalling'', and ''spallation'' have been adopted by particle physicists; in neutron scattering instruments, neutrons are generated by bombarding a uranium (or other) target with a stream of atoms. The neutrons that are ejected from the target are known as "spall". Mechanical spalling Mechanical spalling occurs at high-stress contact points, for example, in a ball bearing. Spalling occurs in preference to brinelling, where the maximal shear stress occurs not at the surface, but just below, shearing the spall off. One of the simplest forms of mechanical spalling is plate impact, in which two waves of compres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversible upon removing the stress. Ductility is a critical mechanical performance indicator, particularly in applications that require materials to bend, stretch, or deform in other ways without breaking. The extent of ductility can be quantitatively assessed using the percent elongation at break, given by the equation: \% \mathrm= \left ( \frac \right )\times100 where l_ is the length of the material after fracture and l_0 is the original length before testing. This formula helps in quantifying how much a material can stretch under tensile stress before failure, providing key insights into its ductile behavior. Ductility is an important consideration in engineering and manufacturing. It defines a material's suitabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can Permeation, permeate solid metals. Once absorbed, hydrogen lowers the Stress (mechanics), stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs in steels, as well as in iron, nickel, titanium, cobalt, and their alloys. Copper, aluminium, and stainless steels are less susceptible to hydrogen embrittlement. The essential facts about the nature of hydrogen embrittlement have been known since the 19th century. Hydrogen embrittlement is maximised at around room temperature in steels, and most metals are relatively immune to hydrogen embrittlement at temperatures above 150 °C. Hydrogen embrittlement requires the presence of both atomic ("diffusible") hydrogen and a Stress (mechanics), mechanical stress to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anodized Titanium Colors
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called ''anodizing'' because the part to be treated forms the anode electrode of an electrolytic cell. Anodizing increases resistance to corrosion and wear, and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add reflected light wave interference effects. Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitors. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium, zinc, magnesium, niobium, zirconium, hafnium, and tantalum. Iron or carbon steel metal exfoliates when oxidized under neutral or alkaline micro-electrolytic condition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quenching
In materials science, quenching is the rapid cooling of a workpiece in water, gas, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring. It does this by reducing the window of time during which these undesired reactions are both thermodynamically favorable and kinetically accessible; for instance, quenching can reduce the crystal grain size of both metallic and plastic materials, increasing their hardness. In metallurgy, quenching is most commonly used to harden steel by inducing a martensite transformation, where the steel must be rapidly cooled through its eutectoid point, the temperature at which austenite becomes unstable. Rapid cooling prevents the formation of cementite structure, instead forcibly dissolving carbon atoms in the ferrite lattice. In steel alloyed with metals such as nickel and manganese, the eutectoid t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |