|
Thrombospondin-1
Thrombospondin 1, abbreviated as THBS1, is a protein that in humans is encoded by the ''THBS1'' gene. Thrombospondin 1 is a subunit of a disulfide-linked homotrimeric protein. This protein is an adhesive glycoprotein that mediates cell-to-cell and cell-to-matrix interactions. This protein can bind to fibrinogen, fibronectin, laminin, collagens types V and VII and integrins alpha-V/beta-1. This protein has been shown to play roles in platelet aggregation, angiogenesis, and tumorigenesis. Function The thrombospondin-1 protein is a member of the thrombospondin family. It is a multi-domain matrix glycoprotein that has been shown to be a natural inhibitor of neovascularization and tumorigenesis in healthy tissue. Both positive and negative modulation of endothelial cell adhesion, motility, and growth have been attributed to TSP1. This should not be surprising considering that TSP1 interacts with at least 12 cell adhesion receptors, including CD36, αv integrins, β1 integri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD47
CD47 (Cluster of Differentiation 47) also known as integrin associated protein (IAP) is a transmembrane protein that in humans is encoded by the CD47 gene. CD47 belongs to the immunoglobulin superfamily and partners with membrane integrins and also binds the ligands thrombospondin 1 (TSP-1) and signal-regulatory protein alpha (SIRPα). CD-47 acts as a ''Eat-me signals, don't eat me'' signal to macrophages of the immune system which has made it a potential therapeutic target in some cancers, and more recently, for the treatment of pulmonary fibrosis. CD47 is involved in a range of cellular processes, including apoptosis, cell growth, proliferation, cell adhesion, adhesion, and cell migration, migration. Furthermore, it plays a role in insulin secretion, as well as immune response, immune and angiogenic responses. CD47 is ubiquitously expressed in human cells and has been found to be overexpressed in many different tumor cells. Expression in equine cutaneous tumors has been repor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reelin
Reelin, encoded by the ''RELN'' gene, is a large secreted extracellular matrix glycoprotein that helps regulate processes of neuronal migration and positioning in the developing brain by controlling cell–cell interactions. Besides this important role in early Developmental biology, development, reelin continues to work in the adult brain. It modulates synaptic plasticity by enhancing the induction and maintenance of long-term potentiation. It also stimulates dendrite and dendritic spine development in the hippocampus, and regulates the continuing migration of neuroblasts generated in adult neurogenesis sites of the subventricular zone, subventricular and subgranular zones. It is found not only in the brain but also in the liver, Thyroid, thyroid gland, adrenal gland, fallopian tube, breast and in comparatively lower levels across a range of anatomical regions. Reelin has been suggested to be implicated in pathogenesis of several brain diseases. The expression of the protein has ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VLDLR
The very-low-density-lipoprotein receptor (VLDLR) is a transmembrane lipoprotein receptor of the low-density-lipoprotein (LDL) receptor family. VLDLR shows considerable homology with the members of this lineage. Discovered in 1992 by Tokuo. Yamamoto and Sadao. Takahashi, VLDLR is widely distributed throughout the tissues of the body, including the heart, skeletal muscle, adipose tissue, and the brain, but is absent from the liver. This receptor has an important role in cholesterol uptake, metabolism of apolipoprotein E-containing triacylglycerol-rich lipoproteins, and neuronal migration in the developing brain. In humans, VLDLR is encoded by the ''VLDLR'' gene. Mutations of this gene may lead to a variety of symptoms and diseases, which include type I lissencephaly, cerebellar hypoplasia, and atherosclerosis. Protein structure VLDLR is a member of the low-density-lipoprotein (LDL) receptor family, which is entirely composed of type I transmembrane lipoprotein receptors. All m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasminogen
Plasmin is an important enzyme () present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein (in the zymogen form of plasminogen) is encoded by the ''PLG'' gene. Function Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. Plasmin is also integrally involved in inflammation. It cleaves fibrin, fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsin, belongs to the family of serine proteases. Plasmin is released as a zymogen called plasminogen (PLG) from the liver into the systemic circulation. Two major glycoforms of plasminogen are present in humans - type I plasminogen contains two glycosyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RGD Motif
Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from ''Drosophila'' to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins. The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics, while the peptide itself is used ubiquitously in bioengineering. Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion. Discovery RGD was identified as the minimal recognition sequence within fibronectin required for cell attachment by Ruoslahti and Pierschbacher in the early 1980s. To do this, the authors synthesized various peptides ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxy-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino-terminal
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
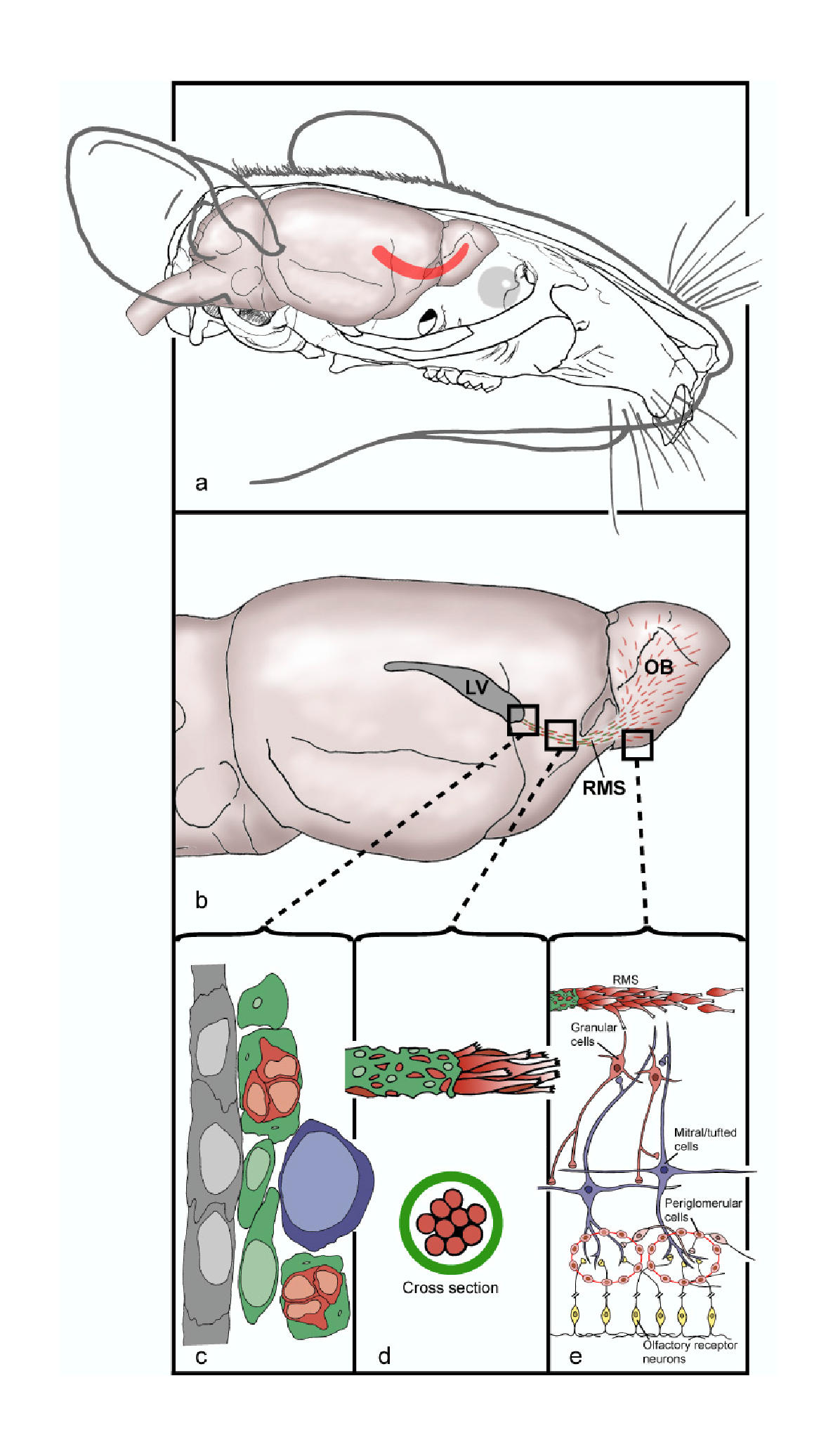
Rostral Migratory Stream
The rostral migratory stream (RMS) is a specialized Cell migration, migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or Periglomerular cell, periglomerular layer. Although it was originally believed that neurons could not regenerate in the adult brain, neurogenesis has been shown to occur in mammalian brains, including those of primates. However, neurogenesis i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ApoER2
Low-density lipoprotein receptor-related protein 8 (LRP8), also known as apolipoprotein E receptor 2 (ApoER2), is a protein that in humans is encoded by the ''LRP8'' gene. ApoER2 is a cell surface receptor that is part of the low-density lipoprotein receptor family. These receptors function in signal transduction and endocytosis of specific ligands. Through interactions with one of its ligands, reelin, ApoER2 plays an important role in embryonic neuronal migration and postnatal long-term potentiation. Another LDL family receptor, VLDLR, also interacts with reelin, and together these two receptors influence brain development and function. Decreased expression of ApoER2 is associated with certain neurological diseases. Structure ApoER2 is a protein made up of 870 amino acids. It is separated into a ligand binding domain of eight ligand binding regions, an EGF-like domain containing three cysteine-rich repeats, an O-linked glycosylation domain of 89 amino acids, a transmembrane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastase
In molecular biology, elastase is an enzyme from the class of proteases (peptidases) that break down proteins, specifically one that can break down elastin. In other words, the name only refers to the substrate specificity (i.e. what proteins it can digest), not to any kind of evolutionary grouping. Forms and classification Eight human genes exist for elastase: The four "pancreatic elastases", chymotrypsin, and neutrophil elastase are serine proteases. The "macrophage elastase" is a matrix metallopeptidase. Chymotrypsin is weaker at digesting elastin than the architypical pancreatic elastase. Some bacteria (including ''Pseudomonas aeruginosa'') also produce elastase; bacterial elestases work in many ways and include serine proteases, aspartic proteases, thiol proteases, and metalloenzymes. Function The fact that elastase can break down elastin in test tubes (while other proteases cannot) does not imply that there is a unifying function for all elastases in the living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathepsin
Cathepsins (Ancient Greek ''kata-'' "down" and ''hepsein'' "boil"; abbreviated CTS) are proteases (enzymes that degrade proteins) found in all animals as well as other organisms. There are approximately a dozen members of this family, which are distinguished by their structure, catalytic mechanism, and which proteins they cleave. Most of the members become activated at the low pH found in lysosomes. Thus, the activity of this family lies almost entirely within those organelles. There are, however, exceptions such as cathepsin K, which works extracellularly after secretion by osteoclasts in bone resorption. Cathepsins have a vital role in mammalian cellular turnover. Classification * Cathepsin A (serine protease) * Cathepsin B (cysteine protease) * Cathepsin C (cysteine protease) * Cathepsin D ( aspartyl protease) * Cathepsin E (aspartyl protease) * Cathepsin F (cysteine proteinase) * Cathepsin G (serine protease) * Cathepsin H (cysteine protease) * Cathepsin K (cyste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |