|
Third Law Of Thermodynamics
The third law of thermodynamics states that the entropy of a closed system at thermodynamic equilibrium approaches a constant value when its temperature approaches absolute zero. This constant value cannot depend on any other parameters characterizing the system, such as pressure or applied magnetic field. At absolute zero (zero kelvins) the system must be in a state with the minimum possible energy. Entropy is related to the number of accessible microstates, and there is typically one unique state (called the ground state) with minimum energy. In such a case, the entropy at absolute zero will be exactly zero. If the system does not have a well-defined order (if its order is glassy, for example), then there may remain some finite entropy as the system is brought to very low temperatures, either because the system becomes locked into a configuration with non-minimal energy or because the minimum energy state is non-unique. The constant value is called the residual entropy of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change and information systems including the transmission of information in telecommunication. Entropy is central to the second law of thermodynamics, which states that the entropy of an isolated system left to spontaneous evolution cannot decrease with time. As a result, isolated systems evolve toward thermodynamic equilibrium, where the entropy is highest. A consequence of the second law of thermodynamics is that certain processes are irreversible. The thermodynami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merle Randall
Merle Randall (January 29, 1888 – March 17, 1950) was an American physical chemist famous for his work with Gilbert N. Lewis, over a period of 25 years, in measuring reaction heat of chemical compounds and determining their corresponding free energy. Together, their 1923 textbook ''"Thermodynamics and the Free Energy of Chemical Substances"'' became a classic work in the field of chemical thermodynamics. In 1932, Merle Randall authored two scientific papers with Mikkel Frandsen: ''"The Standard Electrode Potential of Iron and the Activity Coefficient of Ferrous Chloride,"'' and ''"Determination of the Free Energy of Ferrous Hydroxide from Measurements of Electromotive Force."'' Education Randall completed his Ph.D. at the Massachusetts Institute of Technology in 1912 with a dissertation on ''"Studies in Free Energy"''.Randall, Merle (1912). ''Studies in Free Energy''. Ph.D. Thesis/dissertation — Massachusetts Institute of Technology. Related Based on work by J. Willard ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Moment
In electromagnetism, the magnetic moment or magnetic dipole moment is the combination of strength and orientation of a magnet or other object or system that exerts a magnetic field. The magnetic dipole moment of an object determines the magnitude of torque the object experiences in a given magnetic field. When the same magnetic field is applied, objects with larger magnetic moments experience larger torques. The strength (and direction) of this torque depends not only on the magnitude of the magnetic moment but also on its orientation relative to the direction of the magnetic field. Its direction points from the south pole to the north pole of the magnet (i.e., inside the magnet). The magnetic moment also expresses the magnetic force effect of a magnet. The magnetic field of a magnetic dipole is proportional to its magnetic dipole moment. The dipole component of an object's magnetic field is symmetric about the direction of its magnetic dipole moment, and decreases as the inverse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Ih
Variations in pressure and temperature give rise to different phases of ice, which have varying properties and molecular geometries. Currently, twenty-one phases, including both crystalline and Amorphous solid, amorphous ices have been observed. In modern history, phases have been discovered through scientific research with various techniques including pressurization, force application, nucleation agents, and others. On Earth, most ice is found in the hexagonal Ice Ih phase. Less common phases may be found in the atmosphere and underground due to more extreme pressures and temperatures. Some phases are manufactured by humans for nano scale uses due to their properties. In space, amorphous ice is the most common form as confirmed by observation. Thus, it is theorized to be the most common phase in the universe. Various other phases could be found naturally in astronomical objects. Theory Most liquids under increased pressure freeze at ''higher'' temperatures because the pressu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geometrical Frustration
In condensed matter physics, geometrical frustration (or in short, frustration) is a phenomenon where the combination of conflicting inter-atomic forces leads to complex structures. Frustration can imply a plenitude of distinct ground states at absolute zero, zero temperature, and usual thermal ordering may be suppressed at higher temperatures. Much-studied examples include amorphous materials, glasses, and dilute magnets. The term ''frustration'', in the context of magnetism, magnetic systems, was introduced by Gerard Toulouse in 1977. Frustrated magnetism, magnetic systems had been studied even before. Early work includes a study of the Ising model on a triangular lattice with nearest-neighbor Spin (physics), spins coupled Antiferromagnetism, antiferromagnetically, by Gregory Wannier, G. H. Wannier, published in 1950. Related features occur in magnets with ''competing interactions'', where both ferromagnetic as well as antiferromagnetic couplings between pairs of Spin (physics), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Time-reversal Symmetry
T-symmetry or time reversal symmetry is the theoretical symmetry (physics), symmetry of physical laws under the Transformation (mathematics), transformation of time reversal, : T: t \mapsto -t. Since the second law of thermodynamics states that entropy increases as time flows toward the future, in general, the macroscopic universe does not show symmetry under time reversal. In other words, time is said to be non-symmetric, or asymmetric, except for special equilibrium states when the second law of thermodynamics predicts the time symmetry to hold. However, quantum Weak measurement, noninvasive measurements are predicted to violate time symmetry even in equilibrium, contrary to their classical counterparts, although this has not yet been experimentally confirmed. Time ''asymmetries'' (see Arrow of time) generally are caused by one of three categories: # intrinsic to the dynamic physical law (e.g., for the weak force) # due to the Big Bang, initial conditions of the universe (e.g., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin (physics)
Spin is an Intrinsic and extrinsic properties, intrinsic form of angular momentum carried by elementary particles, and thus by List of particles#Composite particles, composite particles such as hadrons, atomic nucleus, atomic nuclei, and atoms. Spin is quantized, and accurate models for the interaction with spin require relativistic quantum mechanics or quantum field theory. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The relativistic spin–statistics theorem connects electron spin quantization to the Pauli exclusion principle: observations of exclusion imply half-integer spin, and observations of half-integer spin imply exclusion. Spin is described mathematically as a vector for some particles such as photons, and as a spinor or bispinor for other particles such as electrons. Sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
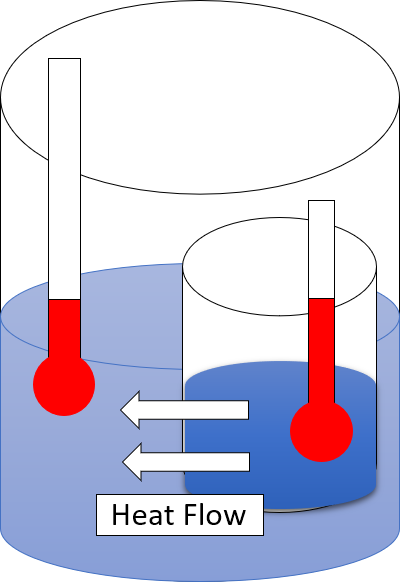
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter (or 'downhill' in terms of the temperature gradient). Another statement is: "Not all heat can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. For example, the first law allows the process of a cup falling off a table and breaking on the floor, as well as allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combinations
In mathematics, a combination is a selection of items from a set that has distinct members, such that the order of selection does not matter (unlike permutations). For example, given three fruits, say an apple, an orange and a pear, there are three combinations of two that can be drawn from this set: an apple and a pear; an apple and an orange; or a pear and an orange. More formally, a ''k''-combination of a set ''S'' is a subset of ''k'' distinct elements of ''S''. So, two combinations are identical if and only if each combination has the same members. (The arrangement of the members in each set does not matter.) If the set has ''n'' elements, the number of ''k''-combinations, denoted by C(n,k) or C^n_k, is equal to the binomial coefficient \binom nk = \frac, which can be written using factorials as \textstyle\frac whenever k\leq n, and which is zero when k>n. This formula can be derived from the fact that each ''k''-combination of a set ''S'' of ''n'' members has k! permutat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Figure Showing Entropy At 0 K
Figure may refer to: General *A shape, drawing, depiction, or geometric configuration *Figure (wood), wood appearance *Figure (music), distinguished from musical motif *Noise figure, in telecommunication *Dance figure, an elementary dance pattern *A person's figure, human physical appearance *Figure–ground (perception), the distinction between a visually perceived object and its surroundings Arts *Figurine, a miniature statuette representation of a creature *Action figure, a posable jointed solid plastic character figurine *Figure painting, realistic representation, especially of the human form *Figure drawing *Model figure, a scale model of a creature Writing *figure, in writing, a type of floating block (text, table, or graphic separate from the main text) *Figure of speech, also called a rhetorical figure *Christ figure, a type of character * in typesetting, text figures and lining figures Accounting *Figure, a synonym for number *Significant figures in a decimal numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |