|
Thermohydraulic
Thermal hydraulics (also called thermohydraulics) is the study of hydraulic flow in thermal fluids. The area can be mainly divided into three parts: thermodynamics, fluid mechanics, and heat transfer, but they are often closely linked to each other. A common example is steam generation in power plants and the associated energy transfer to mechanical motion and the change of states of the water while undergoing this process. Thermal-hydraulics analysis can determine important parameters for reactor design such as plant efficiency and coolability of the system. The common adjectives are "thermohydraulic", "thermal-hydraulics" and "thermalhydraulics". Thermodynamic analysis In the thermodynamic analysis, all states defined in the system are assumed to be in thermodynamic equilibrium; each state has mechanical, thermal, and phase equilibrium, and there is no macroscopic change with respect to time. For the analysis of the system, the first law and second law of thermodynamics can b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydraulics
Hydraulics () is a technology and applied science using engineering, chemistry, and other sciences involving the mechanical properties and use of liquids. At a very basic level, hydraulics is the liquid counterpart of pneumatics, which concerns gases. Fluid mechanics provides the theoretical foundation for hydraulics, which focuses on applied engineering using the properties of fluids. In its fluid power applications, hydraulics is used for the generation, control, and transmission of Power (physics), power by the use of pressure, pressurized liquids. Hydraulic topics range through some parts of science and most of engineering modules, and they cover concepts such as pipe Volumetric flow rate, flow, dam design, fluidics, and fluid control circuitry. The principles of hydraulics are in use naturally in the human body within the vascular system and erectile tissue. ''Free surface hydraulics'' is the branch of hydraulics dealing with free surface flow, such as occurring in rivers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compressor
A compressor is a mechanical device that increases the pressure of a gas by reducing its volume. An air compressor is a specific type of gas compressor. Many compressors can be staged, that is, the gas is compressed several times in steps or stages, to increase discharge pressure. Often, the second stage is physically smaller than the primary stage, to accommodate the already compressed gas without reducing its pressure. Each stage further compresses the gas and increases its pressure and also temperature (if inter cooling between stages is not used). Types Compressors are similar to pumps: both increase the pressure on a fluid (such as a gas) and both can transport the fluid through a pipe. The main distinction is that the focus of a compressor is to change the density or volume of the fluid, which is mostly only achievable on gases. Gases are compressible, while liquids are relatively incompressible, so compressors are rarely used for liquids. The main action of a pump is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Equation
In mathematics and physics (more specifically thermodynamics), the heat equation is a parabolic partial differential equation. The theory of the heat equation was first developed by Joseph Fourier in 1822 for the purpose of modeling how a quantity such as heat diffuses through a given region. Since then, the heat equation and its variants have been found to be fundamental in many parts of both pure and applied mathematics. Definition Given an open subset of and a subinterval of , one says that a function is a solution of the heat equation if : \frac = \frac + \cdots + \frac, where denotes a general point of the domain. It is typical to refer to as time and as spatial variables, even in abstract contexts where these phrases fail to have their intuitive meaning. The collection of spatial variables is often referred to simply as . For any given value of , the right-hand side of the equation is the Laplace operator, Laplacian of the function . As such, the heat equation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
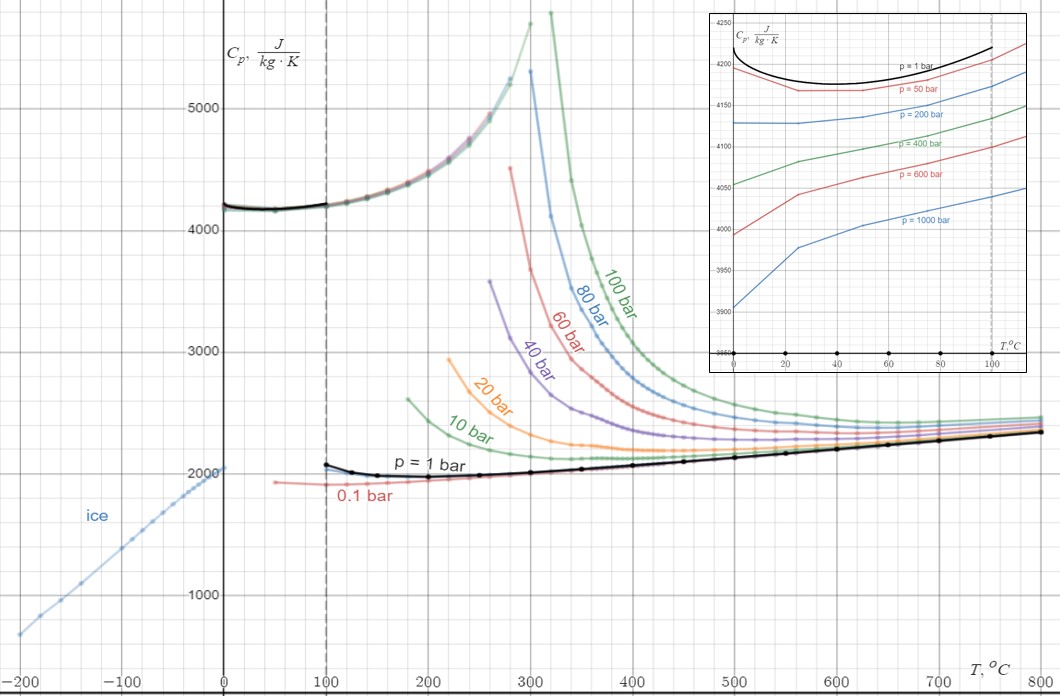
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its '' thermal mass''. Definition Basic definition The heat capacity of an object, denoted by C, is the limit C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per metre squared, or pascal-seconds. Viscosity quantifies the internal friction, frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's center line than near its walls. Experiments show that some stress (physics), stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials such as mineral wool or Styrofoam. Metals have this high thermal conductivity due to free electrons facilitating heat transfer. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rankine Cycle
The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University. Heat energy is supplied to the system via a boiler where the working fluid (typically water) is converted to a high-pressure gaseous state (steam) in order to turn a turbine. After passing over the turbine the fluid is allowed to condense back into a liquid state as waste heat energy is rejected before being returned to boiler, completing the cycle. Friction losses throughout the system are often neglected for the purpose of simplifying calculations as such losses are usually much less significant than thermodynamic losses, especially in larger systems. Description The Rankine cycle closely describes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brayton Cycle
The Brayton cycle, also known as the Joule cycle, is a thermodynamic cycle that describes the operation of certain heat engines that have air or some other gas as their working fluid. It is characterized by isentropic process, isentropic compression and expansion, and isobaric process, isobaric heat addition and rejection, though practical engines have adiabatic process, adiabatic rather than isentropic steps. The most common current application is in airbreathing jet engines and gas turbine engines. The engine cycle is named after George Brayton (1830–1892), the American engineer, who developed the Brayton Ready Motor in 1872, using a piston compressor and piston expander. An engine using the cycle was originally proposed and patented by Englishman John Barber (engineer), John Barber in 1791, using a reciprocating compressor and a turbine expander. There are two main types of Brayton cycles: closed and open. In a closed cycle, the working gas stays inside the engine. Heat i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carnot Cycle
A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem (thermodynamics), Carnot's theorem, it provides an upper limit on the Thermal efficiency, efficiency of any classical Heat engine, thermodynamic engine during the conversion of heat into Work (thermodynamics), work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system. In a Carnot cycle, a Thermodynamic system, system or engine transfers energy in the form of heat between two thermal reservoirs at temperatures T_H and T_C (referred to as the hot and cold reservoirs, respectively), and a part of this transferred energy is converted to the work done by the system. The cycle is Reversible process (thermodynamics), reversible, and entropy is Conserved quantity, conserved, merely transferred between the th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc. For a heat engine, thermal efficiency is the ratio of the net work output to the heat input; in the case of a heat pump, thermal efficiency (known as the '' coefficient of performance'' or COP) is the ratio of net heat output (for heating), or the net heat removed (for cooling) to the energy input (external work). The efficiency of a heat engine is fractional as the output is always less than the input while the COP of a heat pump is more than 1. These values are further restricted by the Carnot theorem. Overview In general, energy conversion efficiency is the ratio between the useful output of a device and the input, in energy terms. For thermal efficiency, the input, Q_, to the device is heat, or the heat-content of a fuel that is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |