|
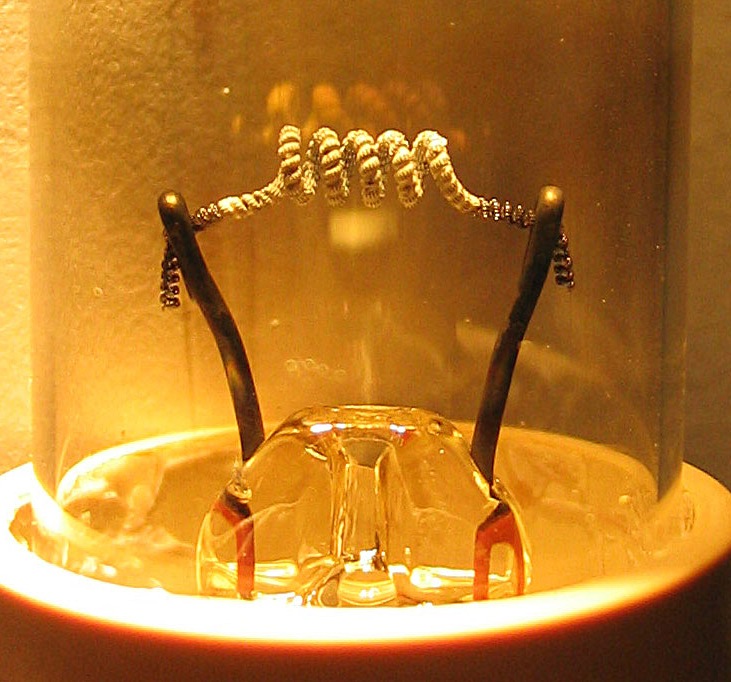
Thermionic Emission
Thermionic emission is the liberation of charged particles from a hot electrode whose thermal energy gives some particles enough kinetic energy to escape the material's surface. The particles, sometimes called ''thermions'' in early literature, are now known to be ions or electrons. Thermal electron emission specifically refers to emission of electrons and occurs when thermal energy overcomes the material's work function. After emission, an opposite charge of equal magnitude to the emitted charge is initially left behind in the emitting region. But if the emitter is connected to a battery, that remaining charge is neutralized by charge supplied by the battery as particles are emitted, so the emitter will have the same charge it had before emission. This facilitates additional emission to sustain an electric current. Thomas Edison in 1880 while inventing his light bulb noticed this current, so subsequent scientists referred to the current as the Edison effect, though it wasn't un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thermionic Filament
Thermionic emission is the liberation of charged particles from a hot electrode whose thermal energy gives some particles enough kinetic energy to escape the material's surface. The particles, sometimes called ''thermions'' in early literature, are now known to be ion source, ions or electrons. Thermal electron emission specifically refers to emission of electrons and occurs when thermal energy overcomes the material's work function. After emission, an opposite charge of equal magnitude to the emitted charge is initially left behind in the emitting region. But if the emitter is connected to a Electric battery, battery, that remaining charge is neutralized by charge supplied by the battery as particles are emitted, so the emitter will have the same charge it had before emission. This facilitates additional emission to sustain an electric current. Thomas Edison in 1880 while inventing Edison light bulb, his light bulb noticed this current, so subsequent scientists referred to the cur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
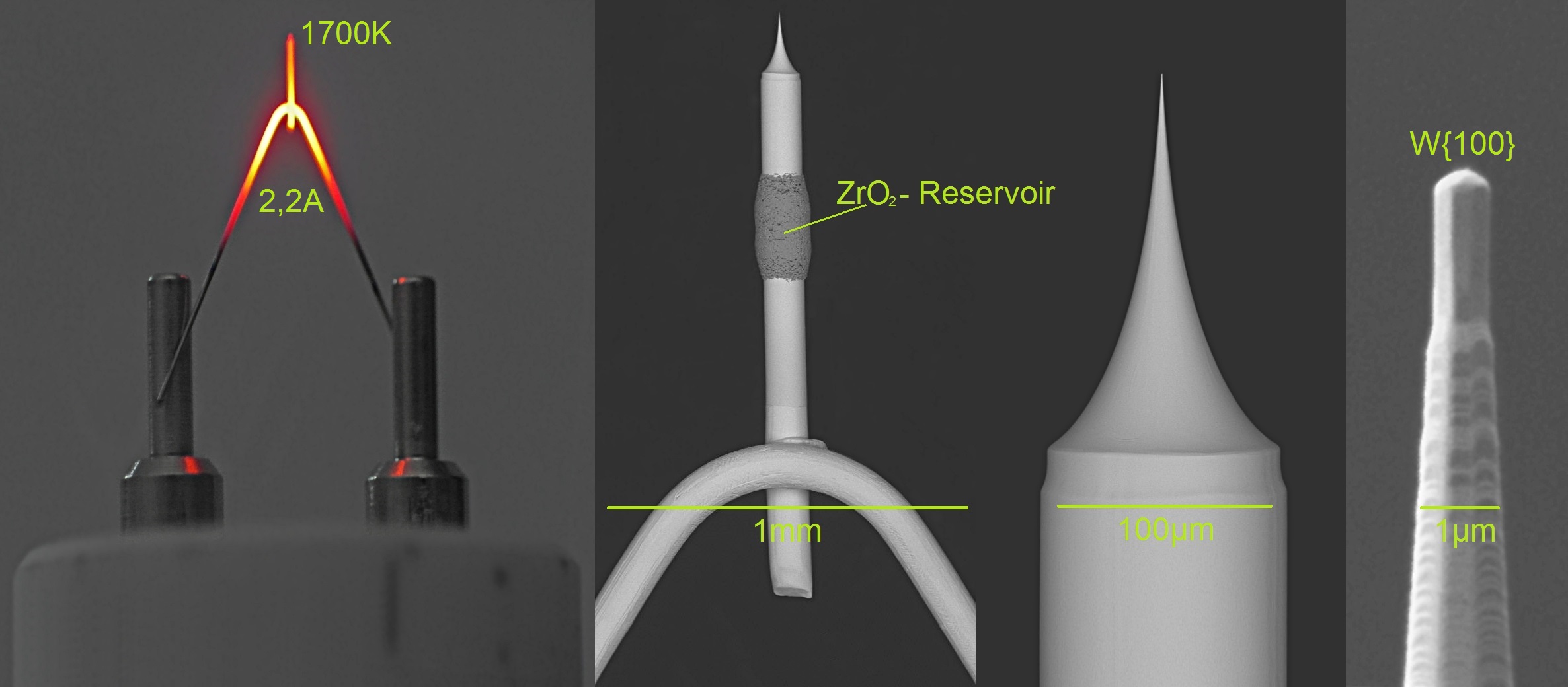
Hot Cathode
In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating element. The heating element is usually an electrical filament heated by a separate electric current passing through it. Hot cathodes typically achieve much higher power density than cold cathodes, emitting significantly more electrons from the same surface area. Cold cathodes rely on field electron emission or secondary electron emission from positive ion bombardment, and do not require heating. There are two types of hot cathode. In a ''directly heated cathode'', the filament is the cathode and emits the electrons. In an ''indirectly heated cathode'', the filament or ''heater'' heats a separate metal cathode electrode which emits the electrons. From the 1920s to the 1960s, a wide variety of electronic devices used hot-cathode vacuum tubes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Johann Wilhelm Hittorf
Johann Wilhelm Hittorf (27 March 1824 – 28 November 1914) was a German physicist who was born in Bonn and died in Münster, Germany. Hittorf was the first to compute the electricity-carrying capacity of charged atoms and molecules ( ions), an important factor for understanding electrochemical reactions. He formulated ion transport numbers and the first method for their measurements. He experimented with tubes containing energy rays extending from a negative electrode. These rays produced a fluorescence when they hit the glass walls of the tubes. In 1876 the effect was named " cathode rays" by Eugen Goldstein. Hittorf's early investigations concerned the allotropes of phosphorus and selenium. Between 1853 and 1859 his most important work concerned ion movement caused by electric current. In 1853 Hittorf revealed that some ions traveled more rapidly than others. This observation resulted in the concept of transport number, the fraction of the electric current carried ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Wexford College Press
Wexford ( ; archaic Yola: ''Weiseforthe'') is the county town of County Wexford, Ireland. Wexford lies on the south side of Wexford Harbour, the estuary of the River Slaney near the southeastern corner of the island of Ireland. The town is linked to Dublin by the M11/N11 National Primary Route; and to Rosslare Europort, Cork and Waterford by the N25. The national rail network connects it to Dublin and Rosslare Europort. It had a population of 21,524 according to the 2022 census. History The town was founded by the Vikings in about 800 AD. They named it ''Veisafjǫrðr'', meaning "inlet of the mudflats". In medieval times, the town was known as ''Weiseforthe'' in the Yola dialect of Middle English. This, in turn became "Wexford" in modern English. According to a story recorded in the '' dindsenchas'', the town's Irish name, ''Loch Garman'' (lake of Garman), comes from a man named '' Garman mac Bomma Licce'' who was chased to the river mouth and drowned as a consequence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Frederick Guthrie (scientist)
Frederick Guthrie FRS FRSE (15 October 1833 – 21 October 1886) was a British physicist, chemist, and academic author. He was the son of Alexander Guthrie, a London tradesman, and the younger brother of mathematician Francis Guthrie. Along with William Fletcher Barrett he founded the Physical Society of London (now the Institute of Physics) in 1874 and was president of the society from 1884 until 1886. He believed that science should be based on experimentation rather than discussion. Academic career His academic career started at University College, London, where he studied for three years. He studied chemistry under Thomas Graham and Alexander William Williamson and mathematics under Augustus De Morgan. In 1852, he submitted his brother Francis's observations to De Morgan. In 1854 Guthrie went to Heidelberg to study under Robert Bunsen and then in 1855 obtained a PhD at the University of Marburg under Adolph Wilhelm Hermann Kolbe. In 1856 he joined Edward Frankland ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Edmond Becquerel
Alexandre-Edmond Becquerel (; 24 March 1820 – 11 May 1891) was a French physicist who studied the solar spectrum, magnetism, electricity, and optics. In 1839, he discovered the photovoltaic effect, the operating principle of the solar cell, which he invented in the same year. He is also known for his work in luminescence and phosphorescence. He was the son of Antoine César Becquerel and the father of Henri Becquerel, the discoverer of radioactivity. Biography Born in Paris, Becquerel was the pupil-turned-successor of his father at the Muséum national d'histoire naturelle. He was also appointed professor at the short-lived Agronomic Institute in Versailles in 1849, and in 1853, received the Chair of Physics at the Conservatoire des arts et métiers. He was associated with his father in much of his work. The first photovoltaic device In 1839, at age 19, while experimenting in his father's laboratory, Becquerel created the world's first photovoltaic cell. In this exper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Solid-state Physics
Solid-state physics is the study of rigid matter, or solids, through methods such as solid-state chemistry, quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. Along with solid-state chemistry, it also has direct applications in the technology of transistors and semiconductors. Background Solid materials are formed from densely packed atoms, which interact intensely. These interactions produce the mechanical (e.g. hardness and Elasticity (physics), elasticity), Heat conduction, thermal, Electrical conduction, electrical, Magnetism, magnetic and Crystal optics, optical properties of solids. Depending on the material involved and the conditions in which it was formed, the atoms may be arranged in a regular, geometric patt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Temperatures
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), with the third being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Metals
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not. Metals are typically ductile (can be drawn into a wire) and malleable (can be shaped via hammering or pressing). A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. The general science of metals is called metallurgy, a subtopic of materials science; aspects of the electronic and thermal properties are also within the scope of condensed matter physics and solid-state chemistry, it is a multidisciplinary topic. In colloquial use materials such as steel alloys are referred to as metals, while others such as polymers, wood or ceramics are nonmetallic materials. A metal conducts electricity at a temperature of absolute z ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Transition Metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. They are lustrous metals with good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Borides
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some borides exhibit very useful physical properties. The term boride is also loosely applied to compounds such as B12As2 (N.B. Arsenic has an electronegativity higher than boron) that is often referred to as icosahedral boride. Ranges of compounds The borides can be classified loosely as boron rich or metal rich, for example the compound YB66 at one extreme through to Nd2Fe14B at the other. The generally accepted definition is that if the ratio of boron atoms to metal atoms is 4:1 or more, the compound is boron rich; if it is less, then it is metal rich. Boron rich borides (B:M 4:1 or more) The main group metals, lanthanides and actinides form a wide variety of boron-rich borides, with metal:boron ratios up to YB66. The properties of this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |