|
Tetrafluoroethylene
Tetrafluoroethylene (TFE) is a fluorocarbon with the chemical formula . It is a colorless gas. Its structure is . It is used primarily in the industrial preparation of fluoropolymers. It is the simplest perfluorinated alkene. It was first reported as "dicarbon tetrafluoride" in 1890. Properties Tetrafluoroethylene is a synthetic colorless, odorless gas that is insoluble in water. Like all unsaturated fluorocarbons, it is susceptible to nucleophilic attack. It is unstable towards decomposition to carbon and carbon tetrafluoride () and prone to form explosive peroxides in contact with air. Industrial use Polymerization of tetrafluoroethylene produces polytetrafluoroethylene (PTFE) polymers such as Teflon and Fluon. PTFE is one of the two fluorocarbon resins composed wholly of fluorine and carbon. The other resin composed purely of carbon and fluorine is the copolymer of TFE with typically 6–9% hexafluoropropene (HFP), which is known as FEP (fluorinated ethylene propylene c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. When used as a lubricant, PTFE reduces fric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," trademarked by the DuPont Company. History In 1938, polytetrafluoroethylene (DuPont brand name Teflon) was discovered by accident by a recently hired DuPont Ph.D., Roy J. Plunkett. While working with tetrafluoroethylene gas to develop refrigerants, he noticed that a previously pressurized cylinder had no pressure remaining. In dissecting the cylinder, he found a mass of white solid in a quantity similar to that of the tetrafluoroethylene gas. It was determined that this material was a new-to-the-world polymer. Tests showed the substance was resistant to corrosion from most acids, bases and solvents and had better high temperature stability than any other plastic. By early 1941, a crash program was making substantial quantities of PT ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a corporate spin-off, spin-off from DuPont (1802–2017), DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances Wetting, wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low polarizability, electric polarizability of fluorine. PTFE has one of the lowest Friction#Coefficient of friction, coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for Cookware and bakeware, pans and other Cookware and bakeware, cookware. It is Chemically inert, non-reactive, partly because of the strength of car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrabromoethylene
Tetrabromoethylene is an organobromine compound with the chemical formula . Its structure is . Under standard conditions, it exists in a form of colorless crystals. It is a brominated derivative of ethylene. Tetrabromoethylene is a potential fungicide and bactericide on fruits. It was used in mineral separation. It is an irritant.https://pubchem.ncbi.nlm.nih.gov/compound/Ethene_-1_1_2_2-tetrabromo It is prepared from acetylene and bromine in multiple steps. One method involves dehydrobromination of pentabromoethane, other method involves bromination of dibromoethylene in chloroform.Miller, S. A. (1965). Acetylene: Its Properties, Manufacture, and Uses. UK Academic Press. Reaction of mercuric acetylide and bromine also gives tetrabromoethylene. It can be produced by oxybrominating butane with free oxygen and bromine. Tetrabromoethylene gives tribromoacetyl bromide upon treatment with fuming nitric acid.Perekalin, V. V. (1964). Unsaturated Nitro Compounds. See also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraiodoethylene
Tetraiodoethylene, TIE or diiodoform, is an Organoiodine chemistry, organoiodine compound with the chemical formula . Its structure is . It is an odourless yellow crystalline solid that is soluble in benzene and chloroform, and insoluble in water.Lide, D. R. (1995). CRC Handbook of Chemistry and Physics. (Special Student Edition): A Ready-Reference Book of Chemical and Physical Data. CRC-Press. Page 522 It has been used as an antiseptic and a component in pesticide and fungicide formulations. It is the periodinated analogue of ethylene. It is a decomposition product of carbon tetraiodide and diiodoacetylene.''Peculiar Decomposition of Diiodoacetylene'', Victor Meyel and Wilhelm Pemsel (1896) Tetraiodoethylene reacts with ethylamine to give ethylamine di-tetraiodoethylene, , and ethylamine tetraiodoethylene, . Tetraiodoethylene and iodine pentafluoride yield iodopentafluoroethane.Journal of the Chemical Society. (1948). page 2188 Tetraiodoethylene turns brown and emits a character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroethane
Hexafluoroethane is an organofluorine compound with the chemical formula . It is a non-flammable colorless odorless gas negligibly soluble in water and slightly soluble in methanol. Its structure is . It is an extremely potent and long-lived greenhouse gas. It is the perfluorocarbon counterpart to the hydrocarbon ethane. Physical properties Hexafluoroethane's Phase (matter), solid phase has two Polymorphism (materials science), polymorphs. In the scientific literature, different phase transition temperatures have been stated. The latest works assign it at 103 K (−170 °C). Below 103 K it has a slightly disordered structure, and over the transition point, it has a Cubic crystal system, body centered cubic structure. The critical point is at 19.89 °C (293.04 K) and 30.39 Bar (unit), bar. Table of densities: Vapor density is 4.823 (air = 1), Relative density, specific gravity at 21 °C is 4.773 (air = 1) and specific volume at 21 °C is 0.1748 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorinated Compound
A perfluorinated compound (PFC) or perfluoro compound is an Organofluorine chemistry, organofluorine compound that lacks C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are Alcohol (chemistry), OH, Perfluorinated carboxylic acid, CO2H, Chlorofluorocarbon, chlorine, perfluoroether, O, and sulfonic acid, SO3H. Electrochemical fluorination, Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds Bioaccumulation, bioaccumulate. Applications One class of perfluorinated compounds, the fluorosurfactants, are widely used in the production of teflon (PTFE) and related fluorinated polymers. They also have been used to confer hydrophobicity and stain-resistance to fabrics. They are components of Firefighting foam, fire-fighting foam. Fluorosurfactants (PFAS) reduce surface tension by concentrating at the liqui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology The word ''pyrolysis'' is coined from the Greek language, Greek-derived morpheme, elements ''pyro-'' (from Ancient Greek : - "fire, heat, fever") and ''lysis'' ( : - "separation, loosening"). Applications Pyrolysis is most commonly used in the treatment of organic compound, organic materials. It is one of the processes involved in the charring of wood or pyrolysis of biomass. In general, pyrolysis of organic substances produces volatile products and leaves Char (chemistry), char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered one of the steps in the processes of gasification or combustion. Laypeople often confuse pyrolysis gas with syngas. Pyrolysis gas has a high percentage of heavy tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or . It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in Developed country, developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene. Production and current applications Worldwide production of R-22 in 2008 was about 800Gg per year, up from about 450Gg per year in 1998, with most production in developing countries. R-22 use is being phased out in developing countries, where it is largely used for air conditioning applications. R-22 is prepared from chloroform: :HCCl3 + 2 HF → HCF2Cl + 2 HCl An important application ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
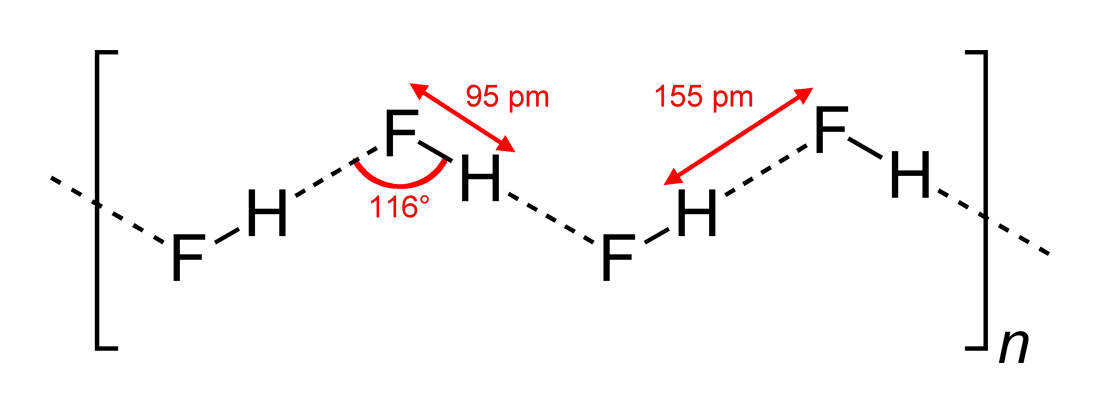
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |