|
Tetraethylammonium Bromide
Tetraethylammonium bromide (TEAB) is a quaternary ammonium compound with the chemical formula C8H20N+Br−, often written as "Et4N+Br−" in the chemical literature. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis. Chemistry Synthesis TEAB is commercially available, but can be prepared by the reaction between tetraethylammonium hydroxide and hydrobromic acid: :Et4N+HO− + HBr → Et4N+Br− + H2O Evaporation of the water and recrystallization from acetonitrile yields a crystalline sample of TEAB. Structure The crystal structure of TEAB has been determined and found to exhibit a distorted tetrahedral symmetry with respect to the geometry of the C atoms around the central N. Synthetic applications Examples include: * TEAB catalyzes the high-yield oxidation of Sulfide_(organic), organic sulfides to sulfoxides by o-iodoxybenzoic acid (IBX) in chloroform/water at room temperatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Compound
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium, ammonium ion () and the primary, secondary, or tertiary ammonium cations, the Quaternary compound, quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salt (chemistry), salts of quaternary ammonium cations. Polyquaternium, Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate viral envelope, enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium
Tetraethylammonium (TEA) is a quaternary ammonium cation with the chemical formula , consisting of four ethyl groups (, denoted Et) attached to a central nitrogen atom. It is a counterion used in the research laboratory to prepare lipophilic salts of inorganic anions. It is used similarly to tetrabutylammonium, the difference being that its salts are less lipophilic, more easily crystallized and more Toxicity, toxic. Preparation The halide salt is prepared by the reaction of triethylamine and an ethyl halide: : This method works well for the preparation of tetraethylammonium iodide (where X = I). Most tetraethylammonium salts are prepared by salt metathesis reactions. For example, the synthesis of tetraethylammonium perchlorate, a salt that has been useful as a supporting electrolyte for polarography, polarographic studies in non-aqueous solvents, is carried out by mixing the water-soluble salts tetraethylammonium bromide and sodium perchlorate in water, from which the water-in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrobromic Acid
Hydrobromic acid is an aqueous solution of hydrogen bromide. It is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. "Constant boiling" hydrobromic acid is an aqueous solution that distills at and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid is one of the strongest mineral acids known. Uses Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. HBr participates in anti-Markovnikov hydrohalogenation of alkenes in the presence of peroxides. The resulting 1-bromoalkanes are versatile alkylating agents, giving rise to fatty amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide (organic)
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern systemati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoxides
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a Chemical polarity, polar functional group. Sulfoxides are oxidized Derivative (chemistry), derivatives of thioether, sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent. Structure and bonding Sulfoxides feature relatively short S–O distances. In DMSO, the S–O distance is 1.531 Å. The sulfur center is pyramidal; the sum of the angles at sulfur is about 306°.. Sulfoxides are generally represented with the structural formula R−S(=O)−R', where R and R' are organic groups. The bond between the sulfur and oxygen atoms is intermediate of a dative bond and a polarized double bond. The double-bond resonance form implies 10 electrons around sulfur (10-S-3 in N-X-L notation). The dou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
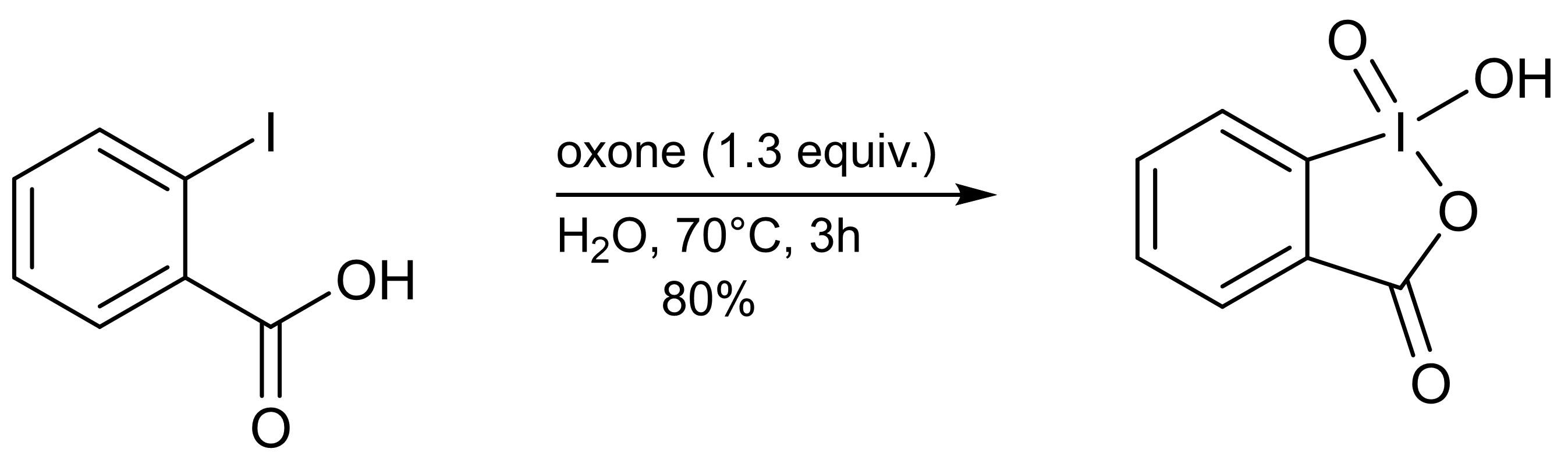
O-iodoxybenzoic Acid
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant such as potassium bromate and sulfuric acid, or more commonly, oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. IBX is an impact- and heat-sensitive explosive (>200°C). Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid. Preparation IBX can be prepared in a single step by adding an excess of oxone to an aqueous solution of 2-iodobenzoic acid. After warming the solution to 70°C for three hours, the precipitated IBX is collected as a white crystalline solid (80% yield, ≥95% purity). Decomposition of IBX to 2-iodosobenzoic acid and 2-iodobenzoic acid occurs at elevated temperatures, and therefore purification by recrystallization f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Superoxide
Potassium superoxide is an inorganic compound with the formula . It is a yellow paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as a scrubber, dehumidifier, and generator in rebreathers, spacecraft, submarines, and spacesuits. Production and reactions Potassium superoxide is produced by burning molten potassium in an atmosphere of excess oxygen. : The salt consists of and ions, linked by ionic bonding. The O–O distance is 1.28 Å. Reactivity Potassium superoxide is a source of superoxide, which is an oxidant and a nucleophile, depending on its reaction partner. Upon contact with water, it undergoes disproportionation to potassium hydroxide, oxygen, and hydrogen peroxide: : : It reacts with carbon dioxide, releasing oxygen: : : Theoretically, 1 kg of absorbs 0.310 kg of while releasing 0.338 kg of . One mole of absorbs 0.5 moles of and releases 0.75 moles of oxygen. Potass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl Halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dialkyl Peroxides
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily breaks, producing free radicals of the form (the dot represents an unpaired electron). Thus, organic peroxides are useful as initiators for some types of polymerization, such as the acrylic, unsaturated polyester, and vinyl ester resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can explosively combust. Organic peroxides, like their inorganic counterparts, are often powerful bleaching agents. Types of organic peroxides Organic peroxides are classified (i) by the presence or absence of a hydroxyl () terminus and (ii) by the presence of alkyl vs acyl substituents. Tert-Butyl hydroperoxide Structural Formula V2.svg, ''tert' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium Chloride
Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula , sometimes written as . In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis. Preparation and structure TEAC is produced by alkylation of triethylamine with ethyl chloride. TEAC exists as either of two stable hydrates, the monohydrate and tetrahydrate. The crystal structure of has been determined, as has that of the tetrahydrate, . Details for the preparation of large, prismatic crystals of are given by Harmon and Gabriele, who carried out Infrared spectroscopy, IR-spectroscopic studies on this and related compounds. These researchers have also pointed out that, although freshly-purified is free of triethylamine hydrochloride, small quantities of this compound form on heating of TEAC as the result of a Hofmann elimination ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium Iodide
Tetraethylammonium iodide is a quaternary ammonium compound with the chemical formula C8H20N+I−. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis. Chemistry Preparation Tetraethylammonium iodide is commercially available, but can be prepared by the reaction between triethylamine and ethyl iodide. Structure The crystal structure of tetraethylammonium iodide has been determined. The crystal structure is a distorted wurtzite lattice. At the nitrogen atom, the coordination is a flattened tetrahedron. The N−C−C angle is slightly larger than the tetrahedral angle. Synthetic applications Examples include: * Stereoselective formation of (Z)-diiodoalkenes by treatment of alkynes with ICl in the presence of tetraethylammonium iodide. * 2-Hydroxyethylation (attachment of −CH2−CH2−OH) by ethylene carbonate of carboxylic acids and certain heterocycles be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethylammonium
Tetraethylammonium (TEA) is a quaternary ammonium cation with the chemical formula , consisting of four ethyl groups (, denoted Et) attached to a central nitrogen atom. It is a counterion used in the research laboratory to prepare lipophilic salts of inorganic anions. It is used similarly to tetrabutylammonium, the difference being that its salts are less lipophilic, more easily crystallized and more Toxicity, toxic. Preparation The halide salt is prepared by the reaction of triethylamine and an ethyl halide: : This method works well for the preparation of tetraethylammonium iodide (where X = I). Most tetraethylammonium salts are prepared by salt metathesis reactions. For example, the synthesis of tetraethylammonium perchlorate, a salt that has been useful as a supporting electrolyte for polarography, polarographic studies in non-aqueous solvents, is carried out by mixing the water-soluble salts tetraethylammonium bromide and sodium perchlorate in water, from which the water-in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |