|
Tantalum Hafnium Carbide
Tantalum hafnium carbide is a refractory chemical compound with a general formula , which can be considered as a solid solution of tantalum carbide and hafnium carbide. It was originally thought to have the highest melting of any known substance but new research has proven that hafnium carbonitride has a higher melting point. Properties Individually, the tantalum and hafnium carbides have the highest melting points among the binary compounds, and , respectively, and their "alloy" with a composition has a melting point of . Very few measurements of melting point in tantalum hafnium carbide have been reported, because of the obvious experimental difficulties at extreme temperatures. A 1965 study of the TaC-HfC solid solutions at temperatures 2,225–2,275 °C found a minimum in the vaporization rate and thus maximum in the thermal stability for . This rate was comparable to that of tungsten and was weakly dependent on the initial density of the samples, which were s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Refractory
In materials science, a refractory (or refractory material) is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the '' refractory metals'', which are elemental metals and their alloys that have high melting temperatures. Refractories are defined by ASTM C71 as "non-metallic materials having those chemical and physical properties that make them applicable for structures, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
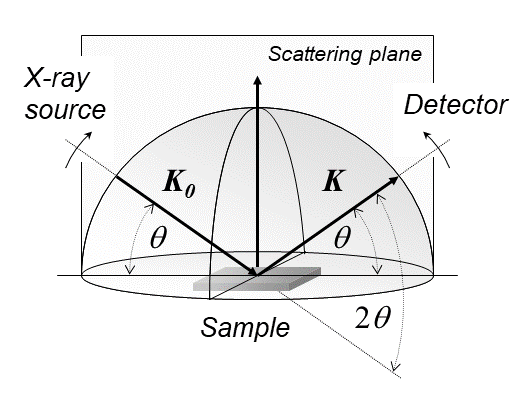
X-ray Diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. The resulting map of the directions of the X-rays far from the sample is called a diffraction pattern. It is different from X-ray crystallography which exploits X-ray diffraction to determine the arrangement of atoms in materials, and also has other components such as ways to map from experimental diffraction measurements to the positions of atoms. This article provides an overview of X-ray diffraction, starting with the early #History, history of x-rays and the discovery that they have the right spacings to be diffracted by crystals. In many cases these diffraction patterns can be #Introduction to x-ray diffraction theory, Interpreted using a single scattering or kinematical theory with conservation of energy (#Ewald's sphere, wave vecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbides
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece. Interstitial / Metallic carbides The carbides of the group 4, 5 and 6 transition metals (with the exception of chromium) are often described as interstitial compounds. These carbides have metallic properties and are refractory. Some exhibit a range of stoichiometries, being a non-stoichiometric mixture of various carbides arising due to crystal defects. Some of them, including titanium carbide and tungsten carbide, are important industrially and are used to coat metals in cutting tools. The long-held view is that the carbon atoms fit into octahedral interstices in a close-packed metal lattice when the metal atom radius is greater than approximately 135 pm: *When the metal atoms are cubic close-packed, (ccp), then filling all of the octahedral interstices with carbon achieves 1: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Refractory Materials
In materials science, a refractory (or refractory material) is a material that is resistant to Thermal decomposition, decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are Inorganic compound, inorganic, Nonmetal, non-metallic compounds that may be Porosity, porous or non-porous, and their crystallinity varies widely: they may be Crystal, crystalline, polycrystalline, Amorphous solid, amorphous, or Composite material, composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the ''refractory metals'', which are elemental metals and their alloys that have high melting temperatures. Refractories are defined by ASTM International, ASTM C71 as " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hafnium Carbonitride
Hafnium carbonitride (HfCN) is an ultra-high temperature ceramic (UHTC) mixed anion compound composed of hafnium (Hf), carbon (C) and nitrogen (N). molecular dynamics calculations have predicted the HfCN (specifically the HfC''0.75''N''0.22'' phase) to have a melting point of 4,110 ± 62 °C (), the highest known for any material. Another approach based on the artificial neural network machine learning pointed towards a similar composition — HfC''0.76''N''0.24''. Experimental testing conducted in 2020 has confirmed a melting point above , substantiating earlier predictions made with atomistic simulations in 2015. Properties The HfCxN1−x has been assessed to possess the following properties: * Thermal conductivity: ** 19–24 W·m−1·K−1 at room temperature, ** 32–39 W·m−1·K−1 at high temperature and with increased nitrogen content. * Electrical conductivity: (149×104)–(213×104) Ω−1 m−1 * Plasticity limit: * Fusion enthalpy: * Flex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hafnium Carbide
Hafnium carbide () is a chemical compound of hafnium and carbon. Previously the material was estimated to have a melting point of about 3,900 °C. More recent tests have been able to conclusively prove that the substance has an even higher melting point of 3,958 °C exceeding those of tantalum carbide and tantalum hafnium carbide which were both previously estimated to be higher. However, it has a low oxidation resistance, with the oxidation starting at temperatures as low as 430 °C. Experimental testing in 2018 confirmed the higher melting point yielding a result of 3,982 (±30°C) with a small possibility that the melting point may even exceed 4,000°C. Atomistic simulations conducted in 2015 predicted that a similar compound, hafnium carbonitride (HfCN), could have a melting point exceeding even that of hafnium carbide. Experimental evidence gathered in 2020 confirmed that it did indeed have a higher melting point exceeding 4,000 °C, with more recent ab i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tantalum Carbide
Tantalum carbides (TaC) form a family of binary chemical compounds of tantalum and carbon with the empirical formula , where ''x'' usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering. Being important cermet materials, tantalum carbides are commercially used in tool bits for cutting applications and are sometimes added to tungsten carbide alloys. The melting points of tantalum carbides was previously estimated to be about depending on the purity and measurement conditions; this value is among the highest for binary compounds. And only tantalum hafnium carbide was estimated to have a higher melting point of . However new tests have conclusively proven that TaC actually has a melting point of 3,768 °C and both tantalum hafnium carbide and hafnium carbide have higher melting points. Preparation powders of desired comp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nickeline
Nickeline or niccolite is the mineral form of nickel arsenide. The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly. Small quantities of sulfur, iron and cobalt are usually present, and sometimes the arsenic is largely replaced by antimony. This last forms an isomorphous series with breithauptite (nickel antimonide). Etymology and history Medieval miners looking for copper in the German Ore Mountains would sometimes find a red mineral, superficially resembling copper ore. Upon attempting extraction, no copper was produced, and subsequently, the miners would be afflicted with mysterious illness. They blamed a mischievous sprite of German mythology, Nickel (similar to ''Old Nick'') for besetting the copper (German: Kupfer). This German equivalent of "copper-nickel" was used as early as 1694 (other old German synonyms are ''Rotnickelkies'' and ''Arsennickel''). In 1751, Baron Axel Fredrik Crons ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |