|
Superexchange
Superexchange or Kramers–Anderson superexchange interaction, is a prototypical ''indirect'' exchange coupling between neighboring magnetic moments (usually next-nearest neighboring cations, see the schematic illustration of MnO below) by virtue of exchanging electrons through a non-magnetic anion known as the superexchange center. In this way, it differs from ''direct'' exchange, in which there is direct overlap of electron wave function from nearest neighboring cations not involving an intermediary anion or exchange center. While direct exchange can be either ferromagnetic or antiferromagnetic, the superexchange interaction is usually antiferromagnetic, preferring opposite alignment of the connected magnetic moments. Similar to the direct exchange, superexchange calls for the combined effect of Pauli exclusion principle and Coulomb's repulsion of the electrons. If the superexchange center and the magnetic moments it connects to are non-collinear, namely the atomic bonds are cant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antisymmetric Exchange
In Physics, antisymmetric exchange, also known as the Dzyaloshinskii–Moriya interaction (DMI), is a contribution to the total magnetic exchange interaction between two neighboring magnetic spins, \mathbf_i and \mathbf_j . Quantitatively, it is a term in the Hamiltonian which can be written as : H^_=\mathbf_ \cdot ( \mathbf_i \times \mathbf_j ). In magnetically ordered systems, it favors a spin canting of otherwise parallel or antiparallel aligned magnetic moments and thus, is a source of weak ferromagnetic behavior in an antiferromagnet. The interaction is fundamental to the production of magnetic skyrmions and explains the magnetoelectric effects in a class of materials termed multiferroics. History The discovery of antisymmetric exchange originated in the early 20th century from the controversial observation of weak ferromagnetism in typically antiferromagnetic -FeO crystals. In 1958, Igor Dzyaloshinskii provided evidence that the interaction was due to the relativisti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Exchange
The double-exchange mechanism is a type of a magnetic exchange that may arise between ions in different oxidation states. First proposed by Clarence Zener, this theory predicts the relative ease with which an electron may be exchanged between two species and has important implications for whether materials are ferromagnetic Ferromagnetism is a property of certain materials (such as iron) that results in a significant, observable magnetic permeability, and in many cases, a significant magnetic coercivity, allowing the material to form a permanent magnet. Ferromagne ..., antiferromagnetic, or exhibit spiral magnetism. For example, consider the 180 degree interaction of Mn- O-Mn in which the Mn "eg" orbitals are directly interacting with the O "2p" orbitals, and one of the Mn ions has more electrons than the other. In the ground state, electrons on each Mn ion are aligned according to the Hund's rule: If O gives up its spin-up electron to Mn4+, its vacant orbital can then b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hendrik Anthony Kramers
Hendrik Anthony "Hans" Kramers (17 December 1894 – 24 April 1952) was a Dutch physicist who worked with Niels Bohr to understand how electromagnetic waves interact with matter and made important contributions to quantum mechanics and statistical physics. Background and education Hans Kramers was born on 17 December 1894 in Rotterdam. the son of Hendrik Kramers, a physician, and Jeanne Susanne Breukelman. In 1912 Hans finished secondary education ( HBS) in Rotterdam, and studied mathematics and physics at the University of Leiden, where he obtained a master's degree in 1916. Kramers wanted to obtain foreign experience during his doctoral research, but his first choice of supervisor, Max Born in Göttingen, was not reachable because of the First World War. Because Denmark was neutral in this war, as was the Netherlands, he travelled (by ship, overland was impossible) to Copenhagen, where he visited unannounced the then still relatively unknown Niels Bohr. Bohr took him on as a P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exchange Interaction
In chemistry and physics, the exchange interaction is a quantum mechanical constraint on the states of indistinguishable particles. While sometimes called an exchange force, or, in the case of fermions, Pauli repulsion, its consequences cannot always be predicted based on classical ideas of force. Both bosons and fermions can experience the exchange interaction. The wave function of identical particles, indistinguishable particles is subject to exchange symmetry: the wave function either changes sign (for fermions) or remains unchanged (for bosons) when two particles are exchanged. The exchange symmetry alters the Expectation value (quantum mechanics), expectation value of the distance between two indistinguishable particles when their wave functions overlap. For fermions the expectation value of the distance increases, and for bosons it decreases (compared to distinguishable particles). The exchange interaction arises from the combination of exchange symmetry and the Coulomb's l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heisenberg Model (quantum)
The quantum Heisenberg model, developed by Werner Heisenberg, is a statistical mechanical model used in the study of critical points and phase transitions of magnetic systems, in which the spins of the magnetic systems are treated quantum mechanically. It is related to the prototypical Ising model, where at each site of a lattice, a spin \sigma_i \in \ represents a microscopic magnetic dipole to which the magnetic moment is either up or down. Except the coupling between magnetic dipole moments, there is also a multipolar version of Heisenberg model called the multipolar exchange interaction. Overview For quantum mechanical reasons (see exchange interaction or ), the dominant coupling between two dipoles may cause nearest-neighbors to have lowest energy when they are ''aligned''. Under this assumption (so that magnetic interactions only occur between adjacent dipoles) and on a 1-dimensional periodic lattice, the Hamiltonian can be written in the form :\hat H = -J \sum_^ \sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
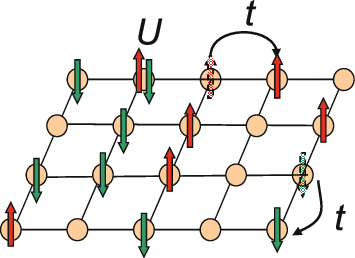
Hubbard Model
The Hubbard model is an Approximation, approximate model used to describe the transition between Conductor (material), conducting and Electrical insulation, insulating systems. It is particularly useful in solid-state physics. The model is named for John Hubbard (physicist), John Hubbard. The Hubbard model states that each electron experiences competing forces: one pushes it to tunnel to neighboring atoms, while the other pushes it away from its neighbors. Its Hamiltonian (quantum mechanics), Hamiltonian thus has two terms: a kinetic term allowing for Quantum tunneling, tunneling ("hopping") of particles between lattice sites and a potential term reflecting on-site interaction. The particles can either be fermions, as in Hubbard's original work, or bosons, in which case the model is referred to as the "Bose–Hubbard model". The Hubbard model is a useful approximation for particles in a periodic potential at sufficiently low temperatures, where all the particles may be assumed t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philip Warren Anderson
Philip Warren Anderson (December 13, 1923 – March 29, 2020) was an American theoretical physicist and Nobel laureate. Anderson made contributions to the theories of Anderson localization, localization, antiferromagnetism, symmetry breaking (including a paper in 1962 discussing symmetry breaking in particle physics, leading to the development of the Standard Model around 10 years later), and high-temperature superconductivity, and to the philosophy of science through his writings on emergent phenomena. Anderson is also responsible for naming the field of physics that is now known as condensed matter physics. Education and early life Anderson was born in Indianapolis, Indiana, and grew up in Urbana, Illinois. His father, Harry Warren Anderson, was a professor of plant pathology at the University of Illinois at Urbana–Champaign, University of Illinois at Urbana-Champaign; his maternal grandfather was a mathematician at Wabash College, where Anderson's father studied; and his m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coulomb's Law
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental scientific law, law of physics that calculates the amount of force (physics), force between two electric charge, electrically charged particles at rest. This electric force is conventionally called the ''electrostatic force'' or Coulomb force. Although the law was known earlier, it was first published in 1785 by French physicist Charles-Augustin de Coulomb. Coulomb's law was essential to the development of the classical electromagnetism, theory of electromagnetism and maybe even its starting point, as it allowed meaningful discussions of the amount of electric charge in a particle. The law states that the magnitude, or absolute value, of the attractive or repulsive electrostatic force between two point Electric charge, charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. Coulomb discovered that bodies with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scalar Product
In mathematics, the dot product or scalar productThe term ''scalar product'' means literally "product with a scalar as a result". It is also used for other symmetric bilinear forms, for example in a pseudo-Euclidean space. Not to be confused with scalar multiplication. is an algebraic operation that takes two equal-length sequences of numbers (usually coordinate vectors), and returns a single number. In Euclidean geometry, the dot product of the Cartesian coordinates of two vectors is widely used. It is often called the inner product (or rarely the projection product) of Euclidean space, even though it is not the only inner product that can be defined on Euclidean space (see ''Inner product space'' for more). It should not be confused with the cross product. Algebraically, the dot product is the sum of the products of the corresponding entries of the two sequences of numbers. Geometrically, it is the product of the Euclidean magnitudes of the two vectors and the cosine of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |