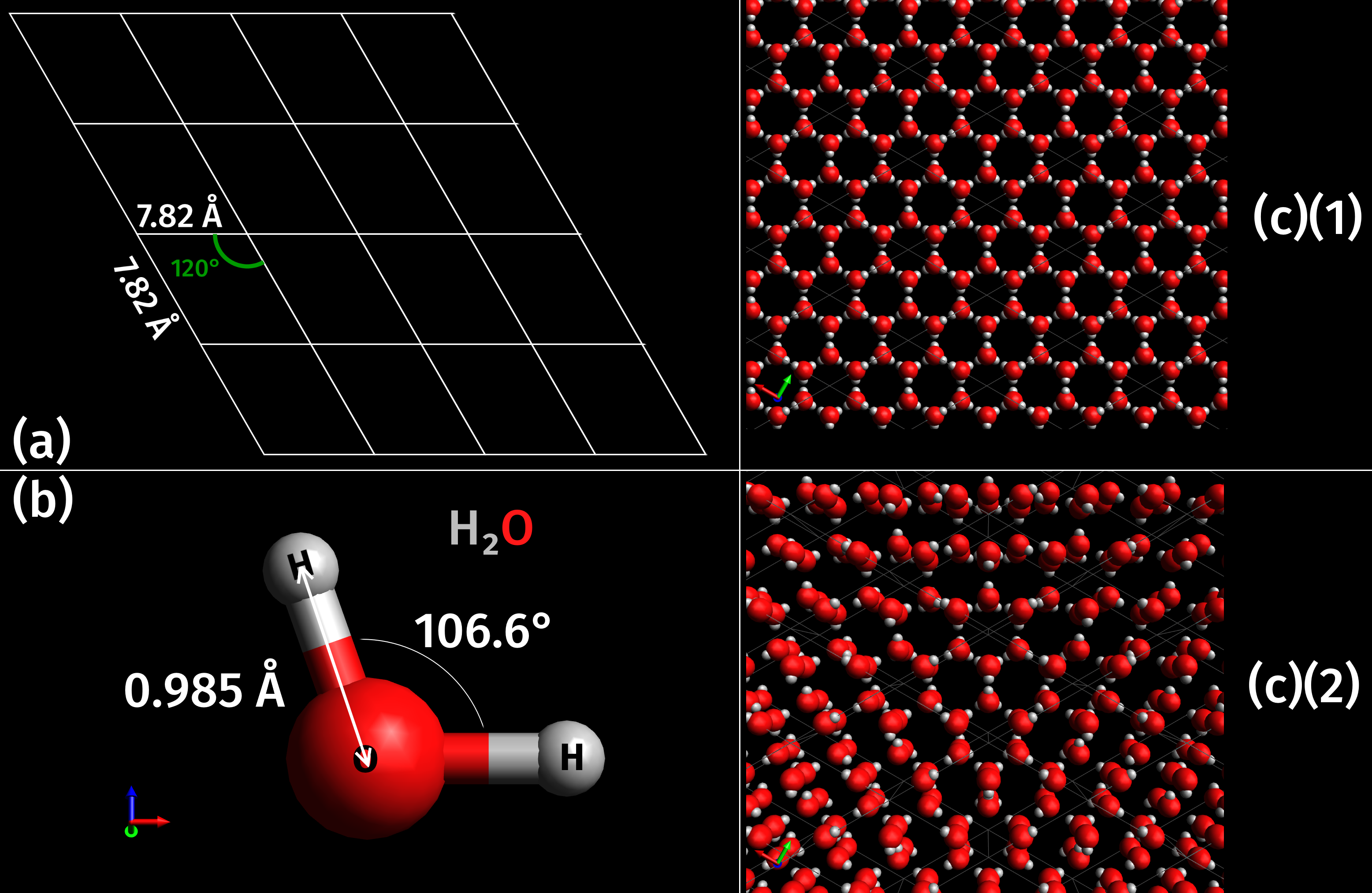
|
Supercool
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. Per the established international definition, supercooling means ''‘cooling a substance below the normal freezing point without solidification’.'' IIR International Dictionary of Refrigeration, http://dictionary.iifiir.org/search.php ASHRAE Terminology, https://www.ashrae.org/technical-resources/free-resources/ashrae-terminology While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to . Supercooled water can occur naturally, for example in the atmosphere, animals or plants. This phenomenon was first identified in 1724 by Daniel Gabriel Fahrenheit, while developing Fahrenheit scale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Transition Temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting temperature, ''T''m, of the crystalline state of the material, if one exists, because the glass is a higher energy state (or enthalpy at const ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleation
In thermodynamics, nucleation is the first step in the formation of either a new Phase (matter), thermodynamic phase or Crystal structure, structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled (at atmospheric pressure) significantly below 0°C, it will tend to Freezing, freeze into ice, but volumes of water cooled only a few degrees below 0°C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay. Nucleation is a common mechanism which generates first-order phase transitions, and it is the start of the process of forming a new thermodynamic phase. In contrast, new phas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing Rain
Freezing rain is rain maintained at temperatures below melting point, freezing by the ambient air mass that causes freezing on contact with surfaces. Unlike rain and snow mixed, a mixture of rain and snow or ice pellets, freezing rain is made entirely of liquid droplets. The raindrops become supercooling, supercooled while passing through a sub-freezing layer of air hundreds of meters above the ground, and then freeze upon impact with any surface they encounter, including the ground, trees, electrical wires, aircraft, and automobiles. The resulting ice, called glaze (ice), glaze ice, can accumulate to a thickness of several centimeters and cover all exposed surfaces. The METAR code for freezing rain is FZRA. A storm that produces a significant thickness of glaze ice from freezing rain is often referred to as an ice storm. Although these storms are not particularly violent, freezing rain is notorious for causing travel problems on roadways, breaking tree limbs, and downing power l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cumulus Cloud
Cumulus clouds are clouds that have flat cloud base, bases and are often described as puffy, cotton-like, or fluffy in appearance. Their name derives from the Latin , meaning "heap" or "pile". Cumulus clouds are low-level clouds, generally less than in altitude unless they are the more vertical cumulus congestus form. Cumulus clouds may appear by themselves, in lines, or in clusters. Cumulus clouds are often precursors of other types of clouds, such as cumulonimbus cloud, cumulonimbus, when influenced by weather factors such as atmospheric instability, instability, humidity, and temperature gradient. Normally, cumulus clouds produce little or no precipitation, but they can grow into the precipitation-bearing cumulus congestus or cumulonimbus clouds. Cumulus clouds can be formed from water vapour, supercooling, supercooled water droplets, or ice crystals, depending upon the ambient temperature. They come in many distinct subforms and generally cool the earth by reflecting the in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Protection System
Ice is water that is frozen into a solid state, typically forming at or below temperatures of 0 ° C, 32 ° F, or 273.15 K. It occurs naturally on Earth, on other planets, in Oort cloud objects, and as interstellar ice. As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. Virtually all of the ice on Earth is of a hexagonal crystalline structure denoted as ''ice Ih'' (spoken as "ice one h"). Depending on temperature and pressure, at least nineteen phases ( packing geometries) can exist. The most common phase transition to ice Ih occurs when liquid water is cooled below (, ) at standard atmospheric pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form. Interstellar ice is overwhelmingly low-density amorphous ice (LD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amorphous Ice
Variations in pressure and temperature give rise to different phases of ice, which have varying properties and molecular geometries. Currently, twenty-one phases, including both crystalline and amorphous ices have been observed. In modern history, phases have been discovered through scientific research with various techniques including pressurization, force application, nucleation agents, and others. On Earth, most ice is found in the hexagonal Ice Ih phase. Less common phases may be found in the atmosphere and underground due to more extreme pressures and temperatures. Some phases are manufactured by humans for nano scale uses due to their properties. In space, amorphous ice is the most common form as confirmed by observation. Thus, it is theorized to be the most common phase in the universe. Various other phases could be found naturally in astronomical objects. Theory Most liquids under increased pressure freeze at ''higher'' temperatures because the pressure helps to hold ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Freezing Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilibrium, equilibrium. The melting point of a substance depends on pressure and is usually specified at a Standard temperature and pressure, standard pressure such as 1 Atmosphere (unit), atmosphere or 100 Pascal (unit), kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to Supercooling, supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the #Melting point measurements, melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystalization
Crystallization is a process that leads to solids with highly organized atoms or molecules, i.e. a crystal. The ordered nature of a crystalline solid can be contrasted with amorphous solids in which atoms or molecules lack regular organization. Crystallization can occur by various routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on factors such as temperature, air pressure, cooling rate, or solute concentration. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. Crystallizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stratus Cloud
Stratus clouds are low-level clouds characterized by horizontal layering with a uniform base, as opposed to convective or cumuliform clouds formed by rising thermals. The term ''stratus'' describes flat, hazy, featureless clouds at low altitudes varying in color from dark gray to nearly white. The word ''stratus'' comes from the Latin prefix ''Strato-'', meaning "layer" or "sheet". Stratus clouds may produce a light drizzle or a small amount of snow. These clouds are essentially above-ground fog formed either through the lifting of morning fog or through cold air moving at low altitudes. Some call these clouds "high fog" for their fog-like form. Formation Stratus clouds form when weak vertical currents lift a layer of air off the ground and it depressurizes, following the lapse rate. This causes the relative humidity to increase due to the adiabatic cooling. This occurs in environments where atmospheric ''stability'' is abundant. Description Stratus clouds look like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |