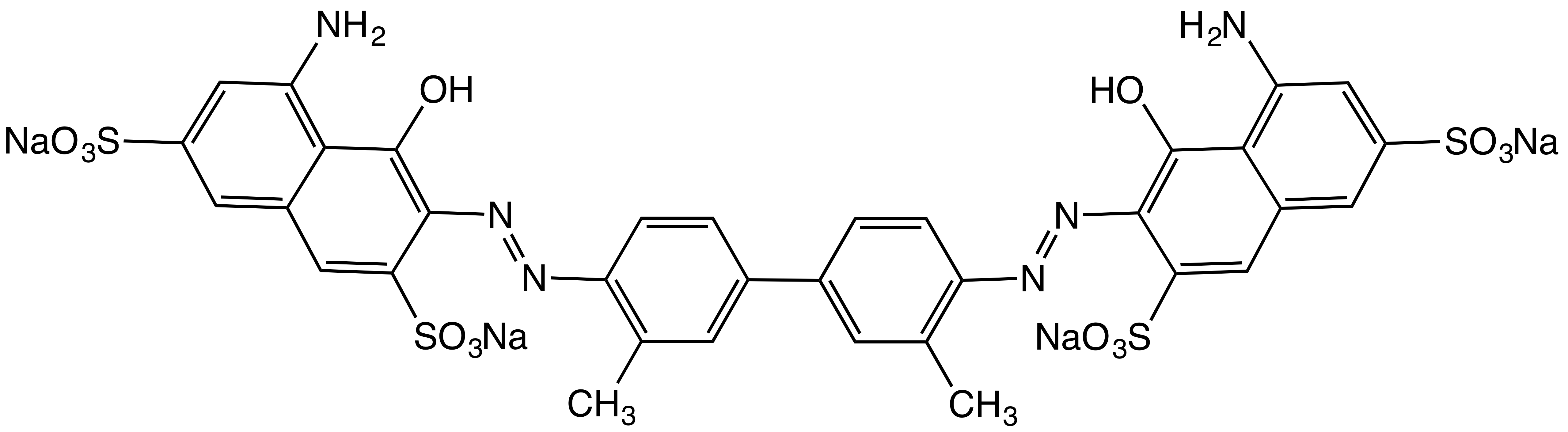
|
Sulfanilic Acid
Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry."Sulphanilic acid". ''A Dictionary of Chemistry''. Oxford University Press, 2000. Oxford Reference Online. Oxford University Press. Synthesis Sulfanilic acid can be produced by sulfonation of aniline with concentrated sulfuric acid.Siegfried Hauptmann: ''Organische Chemie'', 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, . This proceeds via phenylsulfamic acid; a zwitterion with a N-S bond. Eugen Bamberger originally proposed a mechanism involving a series of intramolecular rearrangements, with phenylsulfamic acid forming orthanilic acid, which rearranged to sulfanilic acid on heating. Subsequent radiosulphur studies showed that the process is intermolecular, with the phenylsulfamic acid desulfating to generate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantitative Analysis (chemistry)
In analytical chemistry, quantitative analysis is the determination of the absolute or relative abundance (often expressed as a concentration) of one, several or all particular substance(s) present in a sample. It relates to the determination of percentage of constituents in any given sample. Methods Once the presence of certain substances in a sample is known, the study of their absolute or relative abundance could help in determining specific properties. Knowing the composition of a sample is very important, and several ways have been developed to make it possible, like gravimetric and volumetric analysis. Gravimetric analysis yields more accurate data about the composition of a sample than volumetric analysis but also takes more time to perform in the laboratory. Volumetric analysis, on the other hand, doesn't take that much time and can produce satisfactory results. Volumetric analysis can be simply a titration based in a neutralization reaction but it can also be a prec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Naphthol
2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds. Production Traditionally, 2-naphthol is produced by a two-step process that begins with the sulfonation of naphthalene in sulfuric acid:full-text PDF/ref> :C10H8 + H2SO4 → C10H7SO3H + H2O The sulfonic acid group is then cleaved in molten sodium hydroxide: :C10H7(SO3H) + 3 NaOH → C10H7ONa + Na2SO3 + 2 H2O Neutralization of the product with acid gives 2-naphthol. 2-Naphthol can also be produced by a method analogous to the cumene process. 2-Naphthol-derived dyes The Sudan dyes are popular dyes noted for being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Orange 7
Acid Orange 7, also known as 2-naphthol orange is an azo dye. It is used for dyeing wool. Preparation It is produced by azo coupling of β-naphthol and diazonium derivative of sulfanilic acid Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry."Sulphanili ..... : References Azo dyes Benzenesulfonates Organic sodium salts 2-Naphthols Acid dyes {{OrganicAcid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylaniline
''N'',''N''-Dimethylaniline (DMA) is an organic chemical compound, a substituted derivative of aniline. It is a tertiary amine, featuring a dimethylamino group attached to a phenyl group. This oily liquid is colourless when pure, but commercial samples are often yellow. It is an important precursor to dyes such as crystal violet. Preparation DMA was first reported in 1850 by the German chemist A. W. Hofmann, who prepared it by heating aniline and iodomethane: :C6H5NH2 + 2 CH3I → C6H5N(CH3)2 + 2 HI DMA is produced industrially by alkylation of aniline with methanol in the presence of an acid catalyst:Kahl, Thomas ''et al.'' (2007) "Aniline" in ''Ullmann's Encyclopedia of Industrial Chemistry''. John Wiley & Sons: New York. :C6H5NH2 + 2 CH3OH → C6H5N(CH3)2 + 2 H2O Similarly, it is also prepared using dimethyl ether as the methylating agent. Reactions Dimethylaniline undergoes many of the reactions expected for an aniline, being weakly basic and reactive toward ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Orange
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the p''K''a of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity. Indicator colors In a solution that decreases in acidity, methyl orange moves from the color red to orange and finally to yellow with the opposite occurring for a solution increasing in acidity. This color change from yellow to red occurs because the protons in the acidic solution react with the N=N bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauly Reaction
The Pauly reaction is a chemical test used for detecting the presence of tyrosine or histidine in proteins. It is named after German chemist Hermann Pauly, who first described the reaction. When proteins containing either tyrosine or histidine are reacted with diazotized sulfanilic acid under alkaline conditions, a red color is formed by a coupling reaction In organic chemistry, a coupling reaction is a type of reaction in which two reactant molecules are bonded together. Such reactions often require the aid of a metal catalyst. In one important reaction type, a main group organometallic compound o .... References Protein methods Biochemistry detection reactions {{analytical-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combustion Analysis
Combustion analysis is a method used in both organic chemistry and analytical chemistry to determine the elemental composition (more precisely empirical formula) of a pure organic compound by combusting the sample under conditions where the resulting combustion products can be quantitatively analyzed. Once the number of moles of each combustion product has been determined the empirical formula or a partial empirical formula of the original compound can be calculated. Applications for combustion analysis involve only the elements of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) as combustion of materials containing them convert these elements to their oxidized form (CO2, H2O, NO or NO2, and SO2) under high temperature high oxygen conditions. Notable interests for these elements involve measuring total nitrogen in food or feed to determine protein percentage, measuring sulfur in petroleum products, or measuring total organic carbon (TOC) in water. History The method was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formation Of The Diazonium Derivative Of Sulfanilic Acid
Formation may refer to: Linguistics * Back-formation, the process of creating a new lexeme by removing or affixes * Word formation, the creation of a new word by adding affixes Mathematics and science * Cave formation or speleothem, a secondary mineral deposit formed in a cave * Class formation, a topological group acting on a module satisfying certain conditions * Formation (group theory), a class of groups that is closed under some operations * Formation constant, an equilibrium constant for the formation of a complex in solution * Formation enthalpy, standard heat of formation of a compound * Formation (group theory), a class of groups * Formation (geology), a formally named rock stratum or geological unit * Formation of rocks, how rocks are formed * Formation and evolution of the Solar System, history of the Solar System * Rock formation, an isolated, scenic, or spectacular surface rock outcrop * Vegetation formation, a concept used to classify vegetation communities Milita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colorimetry
Colorimetry is "the science and technology used to quantify and describe physically the human color perception". It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color perception, most often the CIE 1931 XYZ color space tristimulus values and related quantities. History The Duboscq colorimeter was invented by Jules Duboscq in 1870. Instruments Colorimetric equipment is similar to that used in spectrophotometry. Some related equipment is also mentioned for completeness. * A tristimulus colorimeter measures the tristimulus values of a color. * A spectroradiometer measures the absolute spectral radiance (intensity) or irradiance of a light source. * A spectrophotometer measures the spectral reflectance, transmittance, or relative irradiance of a color sample. * A ''spectrocolorimeter'' is a spectrophotometer that can ''calculate'' tristimulus values. * A densitometer measures the degree of lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-(1-Naphthyl)ethylenediamine
''N''-(1-Naphthyl)ethylenediamine is an organic compound. It is commercially available as part of Griess reagents, which find application in quantitative inorganic analysis of nitrates, nitrite and sulfonamide (chemistry), sulfonamide in blood, using the Griess test#Method, Griess test. Preparation This compound can be prepared by the reaction of 1-naphthylamine with 2-chloroethanamine. It is commercially available as the dihydrochloride salt. Properties ''N''-(1-Naphthyl)ethylenediamine undergoes most reactions typical to naphthylamine and primary amines such as diazotation. Similar to its analog ethylenediamine, it can also act as a bidentate ligand to give several coordination compounds. However, it is a weaker bidentate ligand as the nitrogen atom in the naphthylamine group is weakly coordinating due to the dispersal of charge by resonance. For example, it reacts with potassium tetrachloroplatinate in aqueous solution to give (''N''-1-naphthyl-ethylenediamine)-dichloroplatinu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |