|
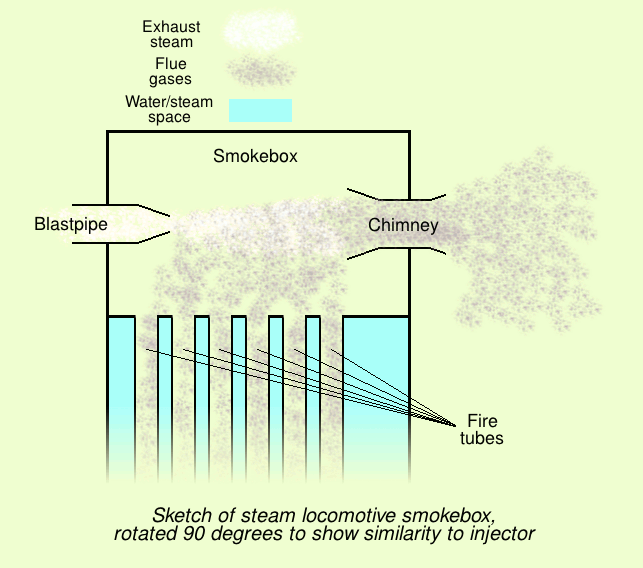
Steam Injector
An injector is a system of ducting and nozzles used to direct the flow of a high-pressure fluid in such a way that a lower pressure fluid is entrained in the jet and carried through a duct to a region of higher pressure. It is a fluid-dynamic pump with no moving parts except a valve to control inlet flow. Depending on the application, an injector can also take the form of an ''eductor-jet pump'', a '' water eductor'' or an ''aspirator''. An '' ejector'' operates on similar principles to create a vacuum feed connection for braking systems etc. The motive fluid may be a liquid, steam or any other gas. The entrained suction fluid may be a gas, a liquid, a slurry, or a dust-laden gas stream. Steam injector The steam injector is a common device used for delivering water to steam boilers, especially in steam locomotives. It is a typical application of the injector principle used to deliver cold water to a boiler against its own pressure, using its own live or exhaust steam, repla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiler Feed Injector Diagram
A boiler is a closed pressure vessel, vessel in which fluid (generally water) is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including Boiler (water heating), water heating, central heating, boiler (power generation), boiler-based power generation, cooking, and sanitation. Heat sources In a fossil fuel power plant using a steam cycle for power generation, the primary heat source will be combustion of Pulverized coal-fired boiler, coal, oil, or natural gas. In some cases byproduct fuel such as the carbon monoxide rich offgasses of a coke battery can be burned to heat a boiler; biofuels such as bagasse, where economically available, can also be used. In a nuclear power plant, boilers called Steam generator (nuclear power), steam generators are heated by the heat produced by nuclear fission. Where a large volume of hot gas is available from some process, a heat recovery steam gener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compressible Flow
Compressible flow (or gas dynamics) is the branch of fluid mechanics that deals with flows having significant changes in fluid density. While all flows are compressibility, compressible, flows are usually treated as being incompressible flow, incompressible when the Mach number (the ratio of the speed of the flow to the speed of sound) is smaller than 0.3 (since the density change due to velocity is about 5% in that case).Anderson, J.D., ''Fundamentals of Aerodynamics'', 4th Ed., McGraw–Hill, 2007. The study of compressible flow is relevant to high-speed aircraft, jet engines, rocket motors, high-speed entry into a planetary atmosphere, gas pipelines, commercial applications such as abrasive blasting, and many other fields. History The study of gas dynamics is often associated with the flight of modern high-speed aircraft and atmospheric reentry of space-exploration vehicles; however, its origins lie with simpler machines. At the beginning of the 19th century, investigation into t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Efficiency
In thermodynamics, the thermal efficiency (\eta_) is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, steam turbine, steam engine, boiler, furnace, refrigerator, ACs etc. For a heat engine, thermal efficiency is the ratio of the net work output to the heat input; in the case of a heat pump, thermal efficiency (known as the '' coefficient of performance'' or COP) is the ratio of net heat output (for heating), or the net heat removed (for cooling) to the energy input (external work). The efficiency of a heat engine is fractional as the output is always less than the input while the COP of a heat pump is more than 1. These values are further restricted by the Carnot theorem. Overview In general, energy conversion efficiency is the ratio between the useful output of a device and the input, in energy terms. For thermal efficiency, the input, Q_, to the device is heat, or the heat-content of a fuel that is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Velocity
Velocity is a measurement of speed in a certain direction of motion. It is a fundamental concept in kinematics, the branch of classical mechanics that describes the motion of physical objects. Velocity is a vector (geometry), vector Physical quantity, quantity, meaning that both magnitude and direction are needed to define it. The Scalar (physics), scalar absolute value (Magnitude (mathematics), magnitude) of velocity is called , being a coherent derived unit whose quantity is measured in the International System of Units, SI (metric system) as metres per second (m/s or m⋅s−1). For example, "5 metres per second" is a scalar, whereas "5 metres per second east" is a vector. If there is a change in speed, direction or both, then the object is said to be undergoing an ''acceleration''. Definition Average velocity The average velocity of an object over a period of time is its Displacement (geometry), change in position, \Delta s, divided by the duration of the period, \Delt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various #Units, units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the International System of Units, SI unit of pressure, the Pascal (unit), pascal (Pa), for example, is one newton (unit), newton per square metre (N/m2); similarly, the Pound (force), pound-force per square inch (Pound per square inch, psi, symbol lbf/in2) is the traditional unit of pressure in the imperial units, imperial and United States customary units, US customary systems. Pressure ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
De Laval Nozzle
A de Laval nozzle (or convergent-divergent nozzle, CD nozzle or con-di nozzle) is a tube which is pinched in the middle, with a rapid convergence and gradual divergence. It is used to accelerate a compressible fluid to supersonic speeds in the axial (thrust) direction, by converting the thermal energy of the flow into kinetic energy. De Laval nozzles are widely used in some types of steam turbines and rocket engine nozzles. It also sees use in supersonic jet engines. Similar flow properties have been applied to jet streams within astrophysics. History Giovanni Battista Venturi designed converging-diverging tubes known as Venturi tubes for experiments on fluid pressure reduction effects when fluid flows through chokes ( Venturi effect). German engineer and inventor Ernst Körting supposedly switched to a converging-diverging nozzle in his steam jet pumps by 1878 after using convergent nozzles but these nozzles remained a company secret. Later, Swedish engineer Gustaf de Lav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Venturi Effect
The Venturi effect is the reduction in fluid pressure that results when a moving fluid speeds up as it flows from one section of a pipe to a smaller section. The Venturi effect is named after its discoverer, the Italian physicist Giovanni Battista Venturi, and was first published in 1797. The effect has various engineering applications, as the reduction in pressure inside the constriction can be used both for measuring the fluid flow and for moving other fluids (e.g. in a vacuum ejector). Background In inviscid fluid dynamics, an incompressible fluid's velocity must ''increase'' as it passes through a constriction in accord with the principle of mass continuity, while its static pressure must ''decrease'' in accord with the principle of conservation of mechanical energy (Bernoulli's principle) or according to the Euler equations. Thus, any gain in kinetic energy a fluid may attain by its increased velocity through a constriction is balanced by a drop in pressure because ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Vaporization
In thermodynamics, the enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. The enthalpy of vaporization is a function of the pressure and temperature at which the transformation (vaporization or evaporation) takes place. The enthalpy of vaporization is often quoted for the normal boiling temperature of the substance. Although tabulated values are usually corrected to 298 K, that correction is often smaller than the uncertainty in the measured value. The heat of vaporization is temperature-dependent, though a constant heat of vaporization can be assumed for small temperature ranges and for reduced temperature . The heat of vaporization diminishes with increasing temperature and it vanishes completely at a certain point called the critical temperature (). Above the critical temperature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (thermodynamics)
Thermodynamic work is one of the principal kinds of process by which a thermodynamic system can interact with and transfer energy to its surroundings. This results in externally measurable macroscopic forces on the system's surroundings, which can cause mechanical work, to lift a weight, for example,Kittel, C. Kroemer, H. (1980). ''Thermal Physics'', second edition, W.H. Freeman, San Francisco, or cause changes in electromagnetic,Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Jackson, J.D. (1975). ''Classical Electrodynamics'', second edition, John Wiley and Sons, New York, .Konopinski, E.J. (1981). ''Electromagnetic Fields and Relativistic Particles'', McGraw-Hill, New York, . or gravitationalNorth, G.R., Erukhimova, T.L. (2009). ''Atmospheric Thermodynamics. Elementary Physics and Chemistry'', Cambridge University Press, Cambridge (UK), . variables. Also, the surroundings can perform t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rankine Cycle
The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University. Heat energy is supplied to the system via a boiler where the working fluid (typically water) is converted to a high-pressure gaseous state (steam) in order to turn a turbine. After passing over the turbine the fluid is allowed to condense back into a liquid state as waste heat energy is rejected before being returned to boiler, completing the cycle. Friction losses throughout the system are often neglected for the purpose of simplifying calculations as such losses are usually much less significant than thermodynamic losses, especially in larger systems. Description The Rankine cycle closely describes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Process
Classical thermodynamics considers three main kinds of thermodynamic processes: (1) changes in a system, (2) cycles in a system, and (3) flow processes. (1) A Thermodynamic process is a process in which the thermodynamic state of a system is changed. A change in a system is defined by a passage from an initial to a final state of thermodynamic equilibrium. In classical thermodynamics, the actual course of the process is not the primary concern, and often is ignored. A state of thermodynamic equilibrium endures unchangingly unless it is interrupted by a thermodynamic operation that initiates a thermodynamic process. The equilibrium states are each respectively fully specified by a suitable set of thermodynamic state variables, that depend only on the current state of the system, not on the path taken by the processes that produce the state. In general, during the actual course of a thermodynamic process, the system may pass through physical states which are not describable as th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |