|
Solanidine
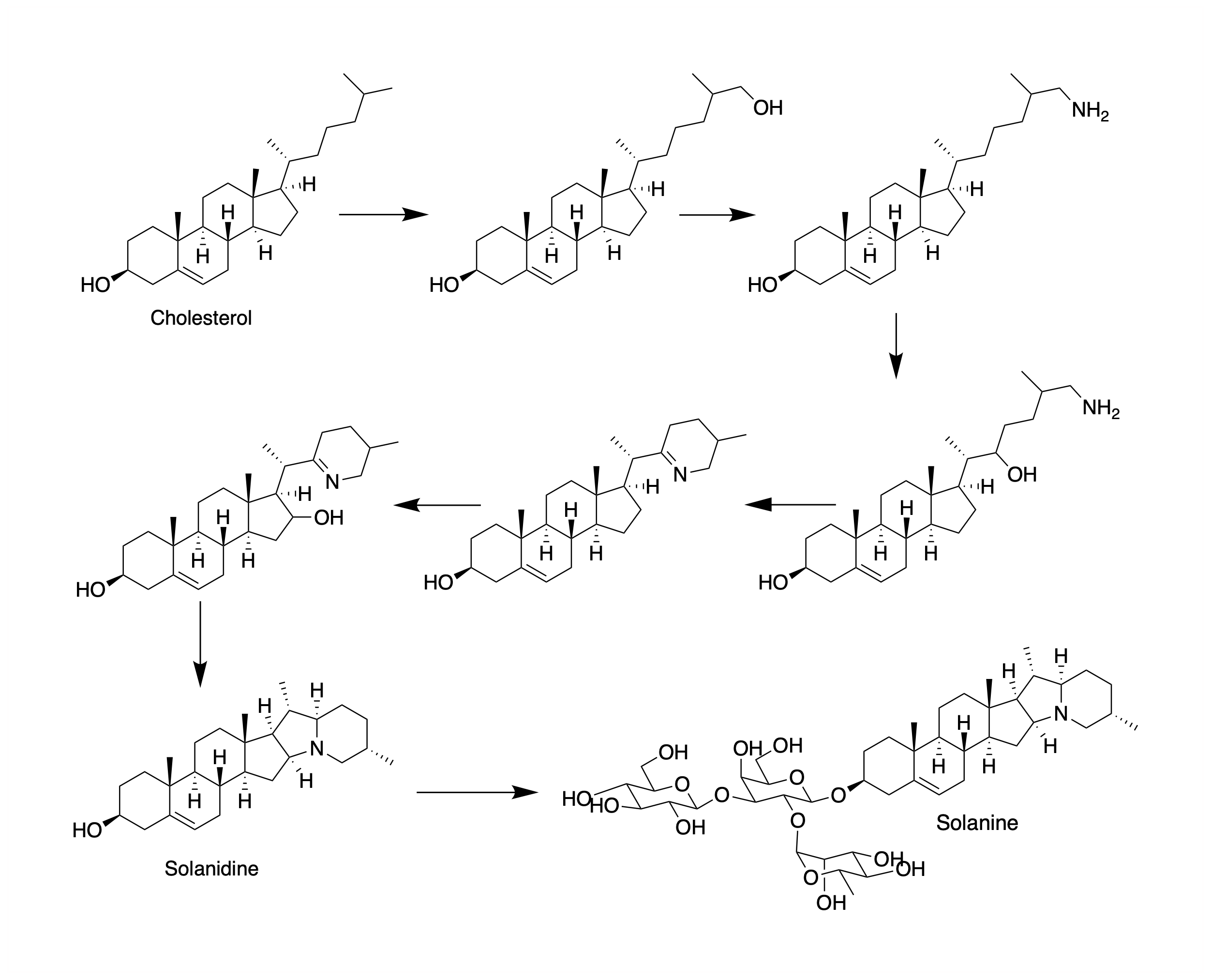
Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and ''Solanum americanum''. Human ingestion of solanidine also occurs via the consumption of the glycoalkaloids, α-solanine and α-chaconine, present in potatoes.Kuiper-Goodman, T., Nawrot, P.S.Solanine and Chaconine IPCS Inchem The sugar portion of these glycoalkaloids hydrolyses in the body, leaving the solanidine portion. Solanidine occurs in the blood serum of normal healthy people who eat potato, and serum solanidine levels fall markedly once potato consumption ceases. Solanidine from food is also stored in the human body for prolonged periods of time, and it has been suggested that it could be released during times of metabolic stress with the potential for deleterious consequences. Solanidine is responsible for neuromuscular syndromes via cholinesterase inhibition.Everist, S.L., ''Poisonous Plants of Australia'', Angus and Robertson, 1974, . U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solanidine To 16-DPA Conversion
Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and ''Solanum americanum''. Human ingestion of solanidine also occurs via the consumption of the glycoalkaloids, α-solanine and α-chaconine, present in potatoes.Kuiper-Goodman, T., Nawrot, P.S.Solanine and Chaconine IPCS Inchem The sugar portion of these glycoalkaloids hydrolyses in the body, leaving the solanidine portion. Solanidine occurs in the blood serum of normal healthy people who eat potato, and serum solanidine levels fall markedly once potato consumption ceases. Solanidine from food is also stored in the human body for prolonged periods of time, and it has been suggested that it could be released during times of metabolic stress with the potential for deleterious consequences. Solanidine is responsible for neuromuscular syndromes via cholinesterase inhibition.Everist, S.L., ''Poisonous Plants of Australia'', Angus and Robertson, 1974, . Uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroidal Alkaloid
Steroidal alkaloids have organic ring backbones which feature nitrogen-based functional groups. More specifically, they are distinguished by their tetracyclic cyclopentanophenanthrene backbone that marks their close relationship with sterols. They fall in two major categories: Solanum alkaloids and Veratrum alkaloids. A Steroidal alkaloid has also been found in ''Chonemorpha fragrans'' (Frangipani vine), 'chonemorphine' was used to treat intestinal infections in Laboratory_rat#Wistar_rat, Wistar rats. (Chatterjee DK et al (1987) Parasitol Res 74, 1, 30-33). ''Solanum'' alkaloids These compounds generally appear as their corresponding glycoside in plants of the Solanum, genus ''Solanum''. ''Solanum'' includes plants like potatoes, tomatoes, and various Solanaceae, nightshades Starting with cholesterol, the biosynthesis of these compounds follow a similar general mechanism including hydroxylation, oxidation, and transamination before differentiating. Alkaloids found in these plants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solanine
Solanine is a glycoalkaloid poison found in species of the nightshade family within the genus ''Solanum'', such as the potato (''Solanum tuberosum''), the tomato (''Solanum lycopersicum''), and the eggplant (''Solanum melongena''). It can occur naturally in any part of the plant, including the leaves, fruit, and tubers. Solanine has pesticidal properties, and it is one of the plant's natural defenses. Solanine was first isolated in 1820 from the berries of the European black nightshade (''Solanum nigrum''), after which it was named. It belongs to the chemical family of saponins. Solanine poisoning Symptoms Solanine poisoning is primarily displayed by gastrointestinal and neurological disorders. Symptoms include nausea, diarrhea, vomiting, stomach cramps, burning of the throat, cardiac dysrhythmia, nightmares, headache, dizziness, itching, eczema, thyroid problems, and inflammation and pain in the joints. In more severe cases, hallucinations, loss of sensation, paraly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
16-DPA
16-Dehydropregnenolone acetate (16-DPA) is a chemical compound used as an intermediate or synthon in the production of many semisynthetic steroids. As 7-ACA is for cephalosporins and 6-APA is for penicillins, 16-DPA is for steroids. While it is not easy to synthesize, it is a convenient intermediate which can be made from other more available materials, and which can then be modified to produce the desired target compound. Upstream sources 16-DPA can be produced from a variety of steroidal sapogenins. Industrially useful sources are diosgenin in mexican yams and solasodine from certain nightshades. These two sapogenins can be used in a one-pot synthesis. Solanidine in potato greens, an alkaloid sapogenin, is also a key source material. Downstream products Compounds derived from 16-DPA include: * Corticosteroids (mainly of a C22 pregnane backbone): hydrocortisone*, betamethasone*, dexamethasone*, beclometasone*, fluticasone, and prednicarbate; * Progestogen (mainly of a C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poisonous
Poison is a chemical substance that has a detrimental effect to life. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broad sense. Whether something is considered a poison may change depending on the amount, the circumstances, and what living things are present. Poisoning could be accidental or deliberate, and if the cause can be identified there may be ways to neutralise the effects or minimise the symptoms. In biology, a poison is a chemical substance causing death, injury or harm to organisms or their parts. In medicine, poisons are a kind of toxin that are delivered passively, not actively. In industry the term may be negative, something to be removed to make a thing safe, or positive, an agent to limit unwanted pests. In ecological terms, poisons introduced into the environment can later cause unwanted effects elsewhere, or in other parts of the food ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Acetate
Mercury(II) acetate is the chemical compound with the formula Hg( O2 CC H3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white water-soluble solid, but samples appear yellowish with time owing to decomposition. Structure Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg. Synthesis and reactions Mercury(II) acetate can be produced by reaction of mercuric oxide with acetic acid. Inorganic reactions Mercury(II) acetate in acetic acid solution reacts with H2S to rapidly precipitate the black (β) polymorph of HgS. With gentle heating of the slurry, the black solid converts to the red form. The mineral cinnabar is red HgS. The precipita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Versus Kinetic Reaction Control
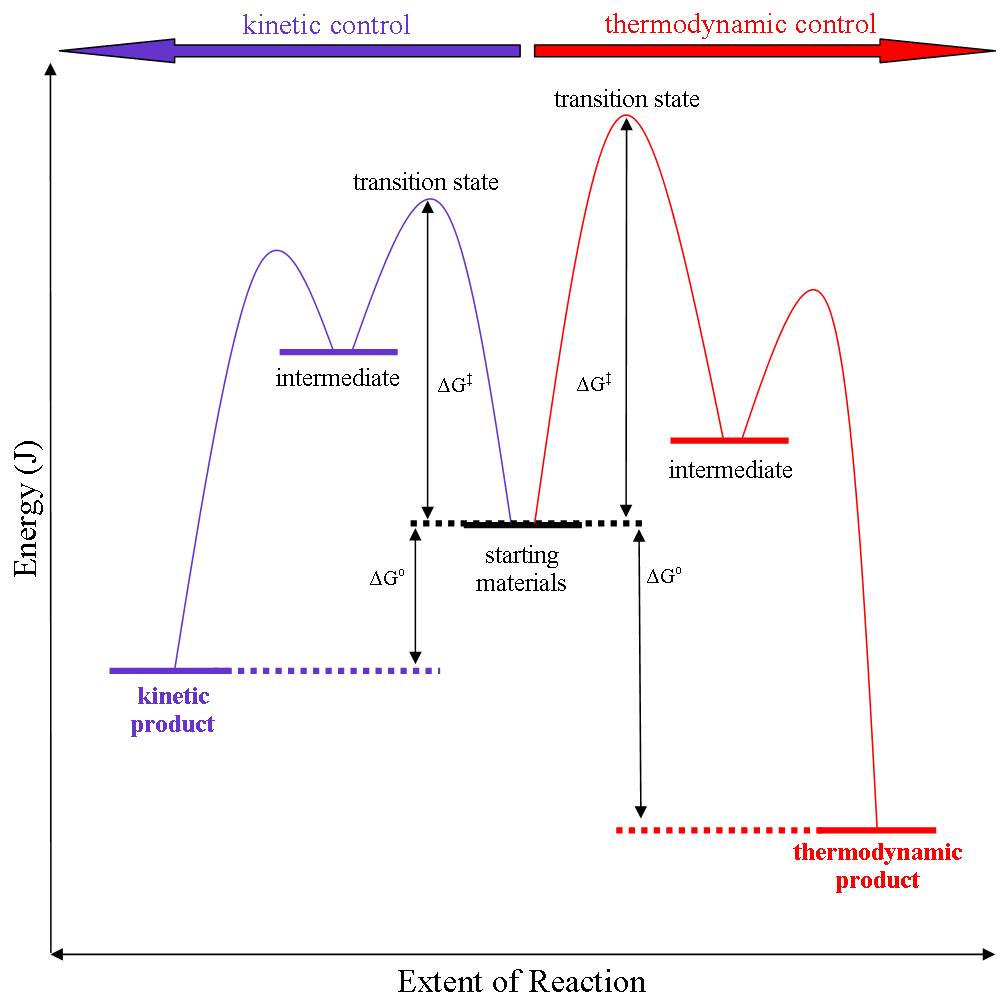
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology. Structure Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with all four substituents. The C=N distances, which are near 129 picometers in length, are shorter than C-N single bonds. Cis/trans isomers are observed. Formation Iminium cations are obtained by protonation and alkylation of imines: :RN=CR'_2 + H+ -> NH=CR'_2 :RN=CR'_2 + R''+ -> R''N=CR'_2 They also are generated by the condensation of secondary amines with ketones or aldehydes: :O=CR'_2 + R2NH + H+ 2N=CR'_2 + H2O This rapid, reversible reaction is one step in "iminium catalysis". More exotic routes to iminium cations are known, e.g. from ring-opening reactions of pyridine. Occurrence Iminium derivatives are common in biology. Pyridoxal phosphate reacts with amino acids to give iminium derivatives. Many iminium salts are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetism, diamagnetic and has a Magnetic susceptibility, diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Chloride
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in 1993. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |