|
Semi-heavy Water
Semiheavy water is the result of replacing one of the protium (normal hydrogen, H) in normal water with deuterium (H; or D). It exists whenever there is water with H and H in the mix. This is because hydrogen atoms (H) are rapidly exchanged between water molecules. Water with 50% H and 50% H, is about 50% HHO and 25% each of HO and HO, in dynamic equilibrium. In normal water, about 1 molecule in 3,200 is HDO (HHO) (one hydrogen in 6,400 is H). By comparison, heavy water DO or HO occurs at a proportion of about 1 molecule in 41 million (i.e., 1 in 6,400). This makes semiheavy water far more common than "normal" heavy water. The freezing point of semiheavy water is close to the freezing point of heavy water at 3.81°C compared to the 3.82°C of heavy water. Production On Earth, semiheavy water occurs naturally in normal water at a proportion of about 1 molecule in 3,200; because 1 in 6,400 hydrogen atoms in water is deuterium, which is 1 part in 3,200 by weight. HDO may be separa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Color Of Water
The color of water varies with the ambient conditions in which that water is present. While relatively small quantities of water appear to be colorless, pure water has a slight blue color that becomes deeper as the thickness of the observed sample increases. The hue of water is an intrinsic property and is caused by selective absorption and scattering of blue light. Dissolved elements or suspended impurities may give water a different color. Intrinsic color The intrinsic color of liquid water may be demonstrated by looking at a white light source through a long pipe that is filled with purified water and closed at both ends with a transparent window. The light sky blue color is caused by weak absorption in the red part of the visible spectrum. Absorptions in the visible spectrum are usually attributed to excitations of electronic energy states in matter. Water is a simple three-atom molecule, H2O, and all its electronic absorptions occur in the ultraviolet region of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protium
Hydrogen (H) has three naturally occurring isotopes: H, H, and H. H and H are stable, while H has a half-life of years. Heavier isotopes also exist; all are synthetic and have a half-life of less than 1 zeptosecond (10 s). Of these, H is the least stable, while H is the most. Hydrogen is the only element whose isotopes have different names that remain in common use today: H is deuterium and H is tritium. The symbols D and T are sometimes used for deuterium and tritium; IUPAC (International Union of Pure and Applied Chemistry) accepts said symbols, but recommends the standard isotopic symbols H and H, to avoid confusion in alphabetic sorting of chemical formulas. H, with no neutrons, may be called protium to disambiguate. (During the early study of radioactivity, some other heavy radioisotopes were given names, but such names are rarely used today.) List of isotopes Note: "y" means year, but "ys" means yoctosecond (10 second). , - , H , 1 , 0 , , colspan=3 alig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more common H has no neutrons. The name ''deuterium'' comes from Greek '' deuteros'', meaning "second". American chemist Harold Urey discovered deuterium in 1931. Urey and others produced samples of heavy water in which the H had been highly concentrated. The discovery of deuterium won Urey a Nobel Prize in 1934. Nearly all deuterium found in nature was synthesized in the Big Bang 13.8 billion years ago, forming the primordial ratio of H to H (~26 deuterium nuclei per 10 hydrogen nuclei). Deuterium is subsequently produced by the slow stellar proton–proton chain, but rapidly destroyed by exothermic fusion reactions. The deuterium–deuterium reaction has the second-lowest energy threshold, and is the most astrophysically acce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction occurs. Substances initially transition between the reactants and products at different rates until the forward and backward reaction rates eventually equalize, meaning there is no net change. Reactants and products are formed at such a rate that the concentration of neither changes. It is a particular example of a system in a steady state. Examples In a new bottle of soda, the concentration of carbon dioxide in the liquid phase has a particular value. If half of the liquid is poured out and the bottle is sealed, carbon dioxide will leave the liquid phase at an ever-decreasing rate, and the partial pressure of carbon dioxide in the gas phase will increase until equilibrium is reached. At that point, due to thermal motion, a molecule of CO2 may leave the liquid phase, but within a very short time another molecule of CO2 will pass from the gas to the liquid, and vice versa. At equilibrium, the rate of tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heavy Water
Heavy water (deuterium oxide, , ) is a form of water (molecule), water in which hydrogen atoms are all deuterium ( or D, also known as ''heavy hydrogen'') rather than the common hydrogen-1 isotope (, also called ''protium'') that makes up most of the hydrogen in normal water. The presence of the heavier isotope gives the water different nuclear properties, and the increase in mass gives it slightly different physical and chemical properties when compared to normal water. Deuterium is a heavy Isotopes of hydrogen, hydrogen isotope. Heavy water contains deuterium atoms and is used in nuclear reactors. Semiheavy water (HDO) is more common than pure heavy water, while heavy-oxygen water is denser but lacks unique properties. Tritiated water is radioactive due to tritium content. Heavy water has different physical properties from regular water, such as being 10.6% denser and having a higher melting point. Heavy water is less Dissociation (chemistry), dissociated at a given temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still. Distillation can operate over a wide range of pressures from 0.14 bar (e.g., ethylbenzene/ styrene) to nearly 21 bar (e.g., propylene/propane) and is capable of separating feeds with high volumetric flowrates and various components that cover a range of relative volatilities from only 1.17 ( o-xylene/ m-xylene) to 81.2 (water/ ethylene glycol). Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity. However, distillation has an enormous environmental footprint, resulting in the consumption of approximately 25% of all industrial energy use. The key issue is that distillation operates based on phase changes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of chemical element, elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity." Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek language, Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure chemical element, elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
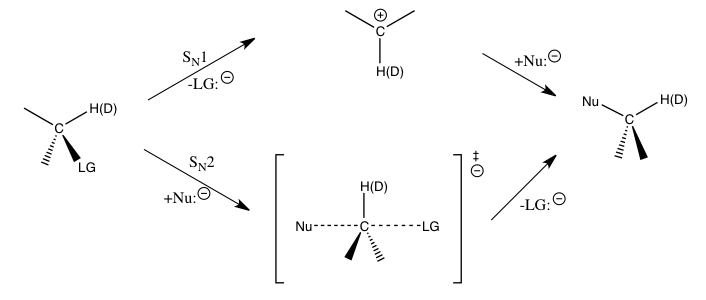
Kinetic Isotope Effect
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (''k'') and the heavy (''k'') isotopically substituted reactants ( isotopologues): KIE = ''k/k''. This change in reaction rate is a quantum effect that occurs mainly because heavier isotopologues have lower vibrational frequencies than their lighter counterparts. In most cases, this implies a greater energy input needed for heavier isotopologues to reach the transition state (or, in rare cases, dissociation limit), and therefore, a slower reaction rate. The study of KIEs can help elucidate reaction mechanisms, and is occasionally exploited in drug development to improve unfavorable pharmacokinetics by protecting metabolically vulnerable C-H bonds. Background KIE is considered one of the most essential ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vienna Standard Mean Ocean Water
Vienna Standard Mean Ocean Water (VSMOW) is an isotopic standard for water, that is, a particular sample of water whose proportions of different isotopes of hydrogen and oxygen are accurately known. VSMOW is distilled from ocean water and does not contain salt or other impurities. Published and distributed by the Vienna-based International Atomic Energy Agency in 1968, the standard and its essentially identical successor, VSMOW2, continue to be used as a reference material. Water samples made up of different isotopes of hydrogen and oxygen have slightly different physical properties. As an extreme example, heavy water, which contains two deuterium (2H) atoms instead of the usual, lighter hydrogen-1 (1H), has a melting point of and boiling point of . Different rates of evaporation cause water samples from different places in the water cycle to contain slightly different ratios of isotopes. Ocean water (richer in heavy isotopes) and rain water (poorer in heavy isotopes) roughly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Isotope Biogeochemistry
Hydrogen isotope biogeochemistry (HIBGC) is the scientific study of biological, geological, and chemical processes in the environment using the distribution and relative abundance of hydrogen isotopes. Hydrogen has two stable isotopes, protium H and deuterium H, which vary in relative abundance on the order of hundreds of Per mille, permil. The ratio between these two species can be called the hydrogen isotopic signature of a substance. Understanding isotopic fingerprints and the sources of Isotope fractionation, fractionation that lead to variation between them can be applied to address a diverse array of questions ranging from ecology and hydrology to geochemistry and paleoclimate reconstructions. Since specialized techniques are required to measure natural hydrogen isotopic composition (HIC), HIBGC provides uniquely specialized tools to more traditional fields like ecology and geochemistry. History of hydrogen isotopes Earliest work The study of hydrogen stable isotopes began ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuterium-depleted Water
Deuterium-depleted water (DDW) is water which has a lower concentration of deuterium than occurs naturally at sea level on Earth. DDW is sometimes known as light water or protium water, although " light water" has long referred to ordinary water, specifically in nuclear reactors. Chemistry Deuterium-depleted water has less deuterium (H) than occurs in nature at sea level. Deuterium is a naturally-occurring, stable (non-radioactive) isotope of hydrogen with a nucleus consisting of one proton and one neutron. A nucleus of normal hydrogen (protium, H) consists of one proton only, and no neutron. Deuterium thus has about twice the atomic mass as H. Heavy water molecules contain two deuteriums instead of two H atoms. The hydrogen in normal water is about 99.97% H (by weight). Production of heavy water involves isolating and removing deuterium-containing isotopologues within natural water. The by-product of this process is DDW. Due to the heterogeneity of hydrological conditions, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Forms Of Water
Form is the shape, visual appearance, or configuration of an object. In a wider sense, the form is the way something happens. Form may also refer to: *Form (document), a document (printed or electronic) with spaces in which to write or enter data *Form (architecture), a combination of external appearance, internal structure, and the unity of the design *Form (education), a class, set, or group of students * Form (religion), an academic term for prescriptions or norms on religious practice *Form, a shallow depression or flattened nest of grass used by a hare *Form, or rap sheet, slang for a criminal record People * Andrew Form, American film producer * Fluent Form, Australian rapper and hip hop musician Arts, entertainment, and media * Form (arts organisation), a Western Australian arts organisation * Form (visual art), a three-dimensional geometrical figure; one of the seven elements of art *Poetic form, a set of structural rules and patterns to which a poem may adhere *Mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |