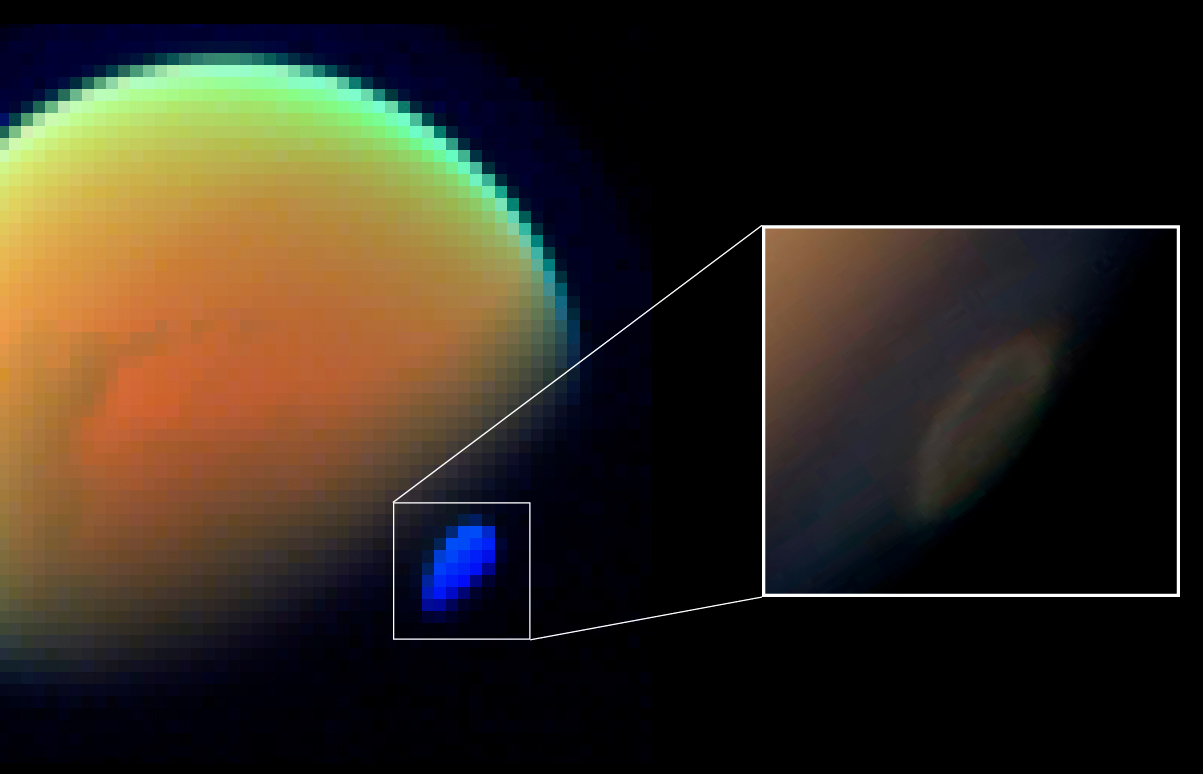
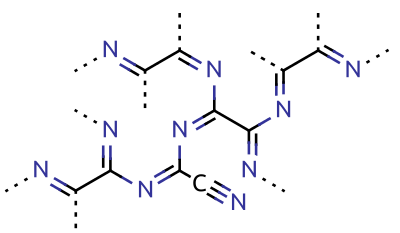
|
Rhodanic Acid
Thiocyanic acid is a chemical compound with the formula and structure , which exists as a tautomer with isothiocyanic acid (). The isothiocyanic acid tautomer tends to dominate with the compound being about 95% isothiocyanic acid in the vapor phase. : It is a moderately strong acid, with a p''K''a of 1.1 at 20 °C and extrapolated to zero ionic strength. One of the thiocyanic acid tautomers, HSCN, is predicted to have a triple bond between carbon and nitrogen. Thiocyanic acid has been observed spectroscopically. The salts and esters of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion () and a suitable cation (e.g., potassium thiocyanate, KSCN). The esters of thiocyanic acid have the general structure , where R stands for an organyl group. Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemical substance, chemicals, pharmaceutical drug, drugs and biomolecule, biologicals with over 10,000 monographs on single substances or groups of related chemical compound, compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Merck Group, Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an append ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued Precursor (chemistry), precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its Volatility (chemistry), volatile nature. A solution of hydrogen cyanide in water (molecule), water, represented as HCN(aqueous, aq), is called ''hydrocyanic acid''. The Salt (chemistry), salts of the cyanide anion are known as cyanides. Whether hydrogen cyanide is an organic compound or not is a topic of debate among chemists, and opinions vary from author to author. Traditionally, it is considered ino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Phase
In physics, a vapor (American English) or vapour (Commonwealth English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Herring, ''General Chemistry'', Prentice-Hall, 8th ed. 2002, p. 483–86. which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas. For example, water has a critical temperature of , which is the highest temperature at which liquid water can exist at any pressure. In the atmosphere at ordinary temperatures gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently. A vapor may co-exist with a liquid (or a solid). When this is true, the two phases will be in equilibrium, and the gas-partial pressure will b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is also shown, either explicitly or implicitly. Unlike other chemical formula types, which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure. For example, many chemical compounds exist in different isomeric forms, which have different enantiomeric structures but the same molecular formula. There are multiple types of ways to draw these structural formulas such as: Lewis structures, condensed formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections. Several systematic chemical naming formats, as in chemical databases, are used that are equivalent to, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen
Cyanogen is the chemical compound with the chemical formula, formula . Its structure is . The simplest stable carbon nitride, it is a Transparency and translucency, colorless and highly toxic gas with a pungency, pungent odor. The molecule is a pseudohalogen. Cyanogen molecules are linear molecular geometry, linear, and consist of two CN groups ‒ analogous to diatomic halogen molecules, such as chlorine, Cl, but far less oxidizing. The two cyanide, cyano groups are bonded together at their carbon atoms, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide () (but see also ''Cyano radical''). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene). Cyanogen is the anhy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolonitrile
Glycolonitrile, also called hydroxyacetonitrile or formaldehyde cyanohydrin, is the organic compound with the formula HOCH2CN. It is the simplest cyanohydrin and it is derived from formaldehyde. It is a colourless liquid that dissolves in water and ether. Because glycolonitrile decomposes readily into formaldehyde and hydrogen cyanide, it is listed as an List of extremely hazardous substances, extremely hazardous substance. In January 2019, astronomers reported the detection of glycolonitrile, another possible Abiogenesis, building block of life among List of interstellar and circumstellar molecules, other such molecules, in outer space. Synthesis and reactions Glycolonitrile is produced by reacting formaldehyde with hydrogen cyanide at near-neutral pH, but with small amounts of catalytic base.Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. Glycolonitrile polymerizes und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoacetonitrile
Aminoacetonitrile is the organic compound with the formula . The compound is a colorless liquid. It is unstable at room temperature, owing to the incompatibility of the amine nucleophile and the nitrile electrophile. For this reason it is usually encountered as the chloride and bisulfate salts of the ammonium derivative, i.e., CCH2NH3sup>+Cl− and CCH2NH3sup>+HSO4−. Production and applications Industrially aminoacetonitrile is produced from glycolonitrile by reaction with ammonia: :HOCH2CN + NH3 → H2NCH2CN + H2O The aminoacetonitrile can be hydrolysed to give glycine: Being bifunctional, it is useful in the synthesis of diverse nitrogen-containing heterocycles. Aminoacetonitrile derivatives are useful antihelmintics. They act as nematode specific ACh agonists causing a spastic paralysis and rapid expulsion from the host. Occurrence in the interstellar medium Using radio astronomy, aminoacetonitrile was discovered in the Large Molecule Heimat, a giant gas cloud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanogen Fluoride
Cyanogen fluoride (IUPAC name: carbononitridic fluoride) is an inorganic compound with the chemical formula . The molecule of this compound is linear, having the structural formula . It consists of a fluorine atom in a single bond with a carbon atom of a cyano group. It is a toxic and explosive gas at room temperature. It is used in organic synthesis and can be produced by pyrolysis of cyanuric fluoride or by fluorination of cyanogen. Synthesis Cyanogen fluoride, is synthesized by the pyrolysis of cyanuric fluoride (C3N3F3) at 1300 °C and 50 mmHg pressure; this process gives a maximum of 50% yield. Other products observed were cyanogen and . For pyrolysis, an induction heated carbon tube with an internal diameter of 0.75 inches is packed with 4 to 8 mesh carbon granules and is surrounded by graphite powder insulation and a water-jacketed shell. The cyanuric fluoride is pyrolyzed (becoming a pyrolysate) at a rate of 50g/hr, and appears as fluffy white solid collected in l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |