|
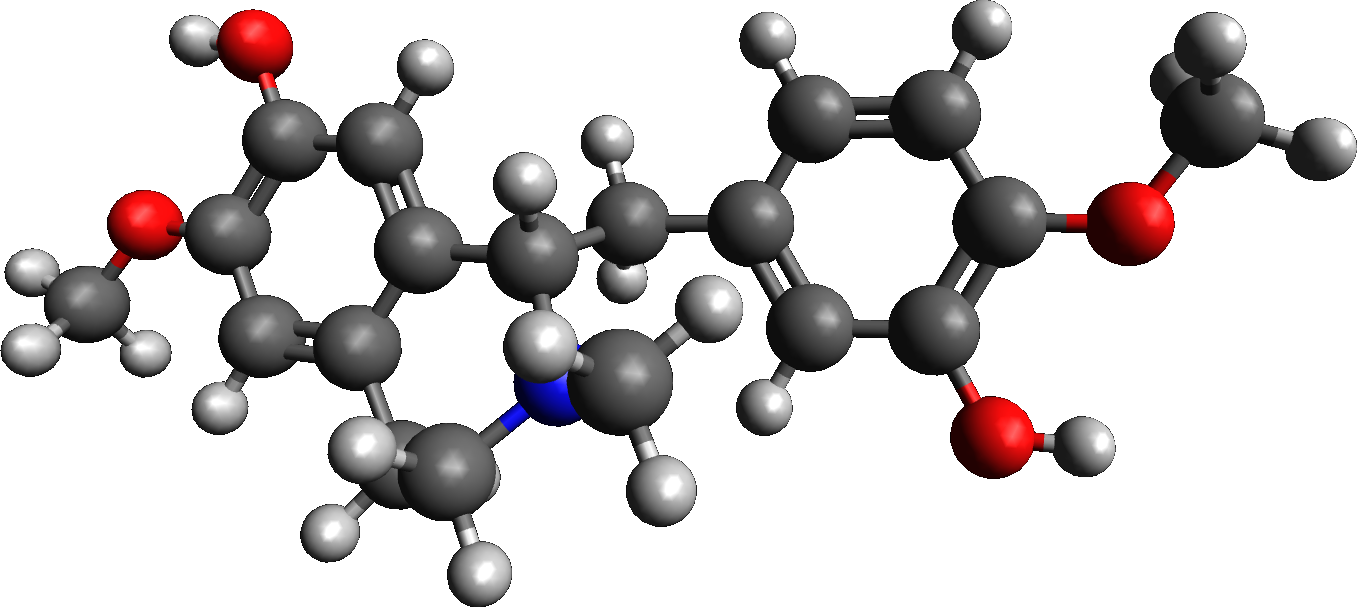
Reticuline
Reticuline is an alkaloid found in opium and a variety of plants including ''Lindera aggregata'', ''Annona squamosa'', and ''Ocotea#Selected species, Ocotea fasciculata'' (also known as ''Ocotea duckei''). Experiments in rodents suggest reticuline possesses potent central nervous system depressant, depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of Parkinson plus syndrome, atypical parkinsonism known as Guadeloupean Parkinsonism. Metabolism 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses S-Adenosyl methionine, ''S''-adenosyl methionine and 3'-hydroxy-''N''-methyl-(''S'')-coclaurine to produce S-Adenosyl-L-homocysteine, ''S''-adenosylhomocysteine and (''S'')-reticuline. Reticuline oxidase uses (''S'')-reticuline and O2 to produce (''S'')-scoulerine and H2O2. Salutaridine synthase uses (''R'')-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticuline 3D
Reticuline is an alkaloid found in opium and a variety of plants including ''Lindera aggregata'', ''Annona squamosa'', and '' Ocotea fasciculata'' (also known as ''Ocotea duckei''). Experiments in rodents suggest reticuline possesses potent central nervous system depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism. Metabolism 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses ''S''-adenosyl methionine and 3'-hydroxy-''N''-methyl-(''S'')- coclaurine to produce ''S''-adenosylhomocysteine and (''S'')-reticuline. Reticuline oxidase uses (''S'')-reticuline and O2 to produce (''S'')- scoulerine and H2O2. Salutaridine synthase uses (''R'')-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine can then be transformed progressively to thebaine, oripavine, and morphine Morphine, formerly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticuline Oxidase
Reticuline is an alkaloid found in opium and a variety of plants including '' Lindera aggregata'', '' Annona squamosa'', and '' Ocotea fasciculata'' (also known as ''Ocotea duckei''). Experiments in rodents suggest reticuline possesses potent central nervous system depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism. Metabolism 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses ''S''-adenosyl methionine and 3'-hydroxy-''N''-methyl-(''S'')- coclaurine to produce ''S''-adenosylhomocysteine and (''S'')-reticuline. Reticuline oxidase uses (''S'')-reticuline and O2 to produce (''S'')- scoulerine and H2O2. Salutaridine synthase uses (''R'')-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine can then be transformed progressively to thebaine, oripavine, and morphine Morphine, forme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salutaridine
Salutaridine, also known as floripavine, is an alkaloid Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids. Alkaloids are produced by a large varie ... that is present in the morphinian alkaloid pathway of opium poppy. Its biosynthetic precursor is the alkaloid (''R'')-reticuline. (''R'')-Reticuline is converted to salutaridine by the enzyme salutaridine synthase. Salutaridine is converted to salutaridinol by the enzyme salutaridine reductase (SalR), with the reduction of NADPH to NADP+. References Morphinans Hydroxyarenes Enones Phenol ethers {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (− O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides, according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to electrophilic aromatic substitutions. Condensation with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Opium Alkaloids
Nature is an inherent character or constitution, particularly of the ecosphere or the universe as a whole. In this general sense nature refers to the laws, elements and phenomena of the physical world, including life. Although humans are part of nature, human activity or humans as a whole are often described as at times at odds, or outright separate and even superior to nature. During the advent of modern scientific method in the last several centuries, nature became the passive reality, organized and moved by divine laws. With the Industrial Revolution, nature increasingly became seen as the part of reality deprived from intentional intervention: it was hence considered as sacred by some traditions (Rousseau, American transcendentalism) or a mere decorum for divine providence or human history (Hegel, Marx). However, a vitalist vision of nature, closer to the pre-Socratic one, got reborn at the same time, especially after Charles Darwin. Within the various uses of the word t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyarenes
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical dru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dehydroreticulinium
Onekama ( ) is a village in Manistee County in the U.S. state of Michigan. The population was 399 at the 2020 census. The village is located on the northeast shore of Portage Lake and is surrounded by Onekama Township. The town's name is derived from ''Ona-ga-maa'', an Anishinaabe word which means "singing water". History The predecessor of the village of Onekama was the settlement of Portage at Portage Point, first established in 1845, at the western end of Portage Lake, at the outlet of Portage Creek. In 1871, when landowners around the land-locked lake became exasperated with the practices of the Portage Sawmill, they took the solution into their own hands and dug a channel through the narrow isthmus, opening a waterway that lowered the lake by and brought it to the same level as Lake Michigan. When this action dried out Portage Creek on May 14, 1871, the settlement, which had only the week before been designated as "Onekama" with a post office under that name, moved to t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dehydroreticulinium Reductase (NADPH)
In enzymology, a 1,2-dehydroreticulinium reductase (NADPH) () is an enzyme that catalyzes the chemical reaction :(R)-reticuline + NADP \rightleftharpoons 1,2-dehydroreticulinium + NADPH + H Thus, the two substrates of this enzyme are (R)-reticuline and NADP, whereas its 3 products are 1,2-dehydroreticulinium, NADPH, and H. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-NH group of donors with NAD+ or NADP+ as acceptor. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivi ... of this enzyme class is (R)-reticuline:NADP+ oxidoreductase. This enzyme is also called 1,2-dehydroreticulinium ion reductase. This enzyme participates in alkaloid biosynthesis i. References * {{Portal bar, Biology, border=no EC 1.5.1 NADP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oripavine
Oripavine is an opioid and the major metabolite of thebaine. It is the precursor to the semi-synthetic compounds etorphine and buprenorphine. Although this chemical compound has analgesic potency comparable to morphine, it is not used clinically due to severe adverse effects and a low therapeutic index. Being a precursor to a series of extremely strong opioids, oripavine is a controlled substance in some jurisdictions. Pharmacological properties Oripavine possesses an analgesic potency comparable to morphine; however, it is not clinically useful due to severe toxicity and low therapeutic index. In both mice and rats, toxic doses caused tonic-clonic seizures followed by death, similar to thebaine. Oripavine has a potential for dependence which is significantly greater than that of thebaine but slightly less than that of morphine. Bridged derivatives (The Bentley compounds) Of much greater relevance are the properties of the orvinols, a large family of semi-synthet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thebaine
Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, '' Thēbai'' (Thebes), an ancient city in Upper Egypt. A minor constituent of opium, thebaine is chemically similar to both morphine and codeine, but has stimulatory rather than depressant effects. At high doses, it causes convulsions similar to strychnine poisoning. The synthetic enantiomer (+)-thebaine does show analgesic effects apparently mediated through opioid receptors, unlike the inactive natural enantiomer (−)-thebaine. While thebaine is not used therapeutically, it is the main alkaloid extracted from '' Papaver bracteatum'' (Iranian opium / Persian poppy) and can be converted industrially into a variety of compounds, including hydrocodone, hydromorphone, oxycodone, oxymorphone, nalbuphine, naloxone, naltrexone, buprenorphine, butorphanol and etorphine. Thebaine is controlled under international law, is listed as a Class A dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salutaridine Synthase
In enzymology, a salutaridine synthase () is an enzyme that catalyzes the chemical reaction :(R)-reticuline + NADPH + H+ + O2 \rightleftharpoons salutaridine + NADP+ + 2 H2O The 4 substrates of this enzyme are (R)-reticuline, NADPH, H+, and O2, whereas its 3 products are salutaridine, NADP+, and H2O. This enzyme belongs to the family of oxidoreductases, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with NADH or NADPH as one donor, and the other dehydrogenated. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivi ... of this enzyme class is (R)-reticuline,NADPH:oxygen oxidoreductase (C-C phenol-coupling). This enzyme is also called (R)-reticuline oxida ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |