|
Radon-222
Radon-222 (222Rn, Rn-222, historically radium emanation or radon) is the most stable isotope of radon, with a half-life of approximately 3.8215(2) days. It is transient in the decay chain of primordial uranium-238 and is the immediate decay product of radium-226. Radon-222 was first observed in 1899, and was identified as an isotope of a new element several years later. In 1957, the name ''radon'', formerly the name of only radon-222, became the name of the element. Owing to its gaseous nature and high radioactivity, radon-222 is one of the leading causes of lung cancer. History Following the 1898 discovery of radium through chemical analysis of radioactive ore, Marie and Pierre Curie observed a new radioactive substance emanating from radium in 1899 that was strongly radioactive for several days. Around the same time, Ernest Rutherford and Robert B. Owens observed a similar (though shorter-lived) emission from thorium compounds. German physicist Friedrich Ernst Dorn exten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radon
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only Rn has a sufficiently long half-life (3.825 days) for it to be released from the soil and rock where it is generated. Radon isotopes are the immediate decay products of radium isotopes. The instability of Rn, its most stable isotope, makes radon one of the rarest elements. Radon will be present on Earth for several billion more years despite its short half-life, because it is constantly being produced as a step in the decay chains of U and Th, both of which are abundant radioactive nuclides with half-lives of at least several billion years. The decay of radon produces many other short-lived nuclides, known as "radon daughters", ending at stable isotopes of lead. Rn occurs in significant quantities as a step in the normal radioactive decay chain of U, also known as the uranium series, which slo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radium-226
Radium-226 () is the longest-lived isotope of radium, with a half-life of 1600 years. It is an intermediate product in the decay chain of uranium-238; as such, it can be found naturally in uranium-containing minerals. Occurrence and decay occurs in the decay chain of uranium-238 (), which is the most common naturally occurring isotope of uranium. It undergoes alpha decay to radon-222, which is also radioactive; the decay chain ultimately terminates at lead-206. Because of its occurrence in the decay chain, exists naturally at low concentrations in uranium-containing minerals, soil, and groundwater. Historical uses Following its discovery by Marie and Pierre Curie in 1898, radium (principally ) has had a number of uses. In the early 20th century, when the hazards of radiation were not well-known, radium was commonly used in consumer items such as toothpaste and hair creams. Radium was also formerly used as a radiation source for cancer treatment, but has since been replac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Radioisotope
A trace radioisotope is a radioisotope that occurs naturally in trace amounts (i.e. extremely small). Generally speaking, trace radioisotopes have half-lives that are short in comparison with the age of the Earth, since primordial nuclides tend to occur in larger than trace amounts. Trace radioisotopes are therefore present only because they are continually produced on Earth by natural processes. Natural processes which produce trace radioisotopes include cosmic ray bombardment of stable nuclides, ordinary alpha and beta decay of the long-lived heavy nuclides, thorium-232, uranium-238, and uranium-235, spontaneous fission of uranium-238, and nuclear transmutation Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed. A transmutat ... reactions induced by natural radioactivity, such as the production of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friedrich Ernst Dorn
Friedrich Ernst Dorn (27 July 1848 – 16 December 1916) was a German physicist. He is best remembered for his discovery that radium emits a radioactive substance, later named radon. Life and work Dorn was born in Guttstadt (Dobre Miasto), Province of Prussia (nowadays Warmia in Poland), and died in Halle, Province of Saxony. He was educated at Königsberg and went on to teach at the university level. In 1885, at Halle University, Dorn took over the position of personal ''ordinarius'' professor for theoretical physics from Anton Oberbeck. Since Dorn was already an ''ordinarius'' professor, he was allowed to assume the title so as to not appear as having been demoted. In 1895, Dorn succeeded Hermann Knoblauch at Halle as the ''ordinarius'' professor for experimental physics and director of the physics institute. Dorn's previous duties were assumed by Carl Schmidt, who had been a Privatdozent and was called as an ''extraordinarius'' professor for theoretical physics. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
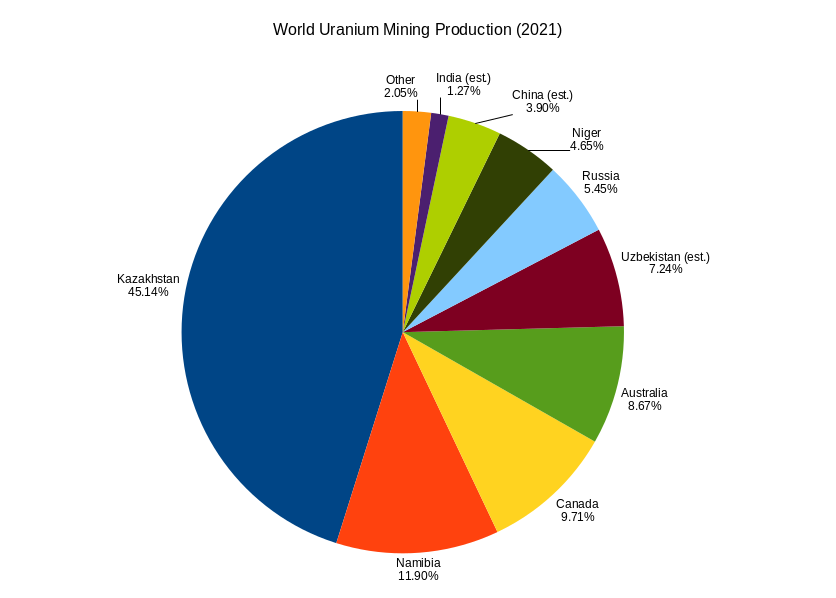
Uranium Mining
Uranium mining is the process of extraction of uranium ore from the earth. Over 50,000 tons of uranium were produced in 2019. Kazakhstan, Canada, and Australia were the top three uranium producers, respectively, and together account for 68% of world production. Other countries producing more than 1,000 tons per year included Namibia, Niger, Russia, Uzbekistan and China. Nearly all of the world's mined uranium is used to power nuclear power plants. Historically uranium was also used in applications such as uranium glass or ferrouranium but those applications have declined due to the radioactivity and toxicity of uranium and are nowadays mostly supplied with a plentiful cheap supply of depleted uranium which is also used in Armour-piercing ammunition, uranium ammunition. In addition to being cheaper, depleted uranium is also less radioactive due to a lower content of short-lived and than natural uranium. Uranium is mined by in-situ leaching (57% of world production) or by convent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum. When used as a Units of energy, unit of energy, the numerical value of 1 eV in joules (symbol J) is equal to the numerical value of the Electric charge, charge of an electron in coulombs (symbol C). Under the 2019 revision of the SI, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in Particle accelerator#Electrostatic particle accelerators, electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V''. Definition and use An electronvolt is the amount of energy gained or lost by a single electron when it moves through an Voltage, electric potential differenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Half-life
Partial may refer to: Mathematics * Partial derivative, derivative with respect to one of several variables of a function, with the other variables held constant ** ∂, a symbol that can denote a partial derivative, sometimes pronounced "partial dee" **Partial differential equation, a differential equation that contains unknown multivariable functions and their partial derivatives Other uses * Partial application, in computer science the process of fixing a number of arguments to a function, producing another function * Partial charge or net atomic charge, in chemistry a charge value that is not an integer or whole number * Partial fingerprint, impression of human fingers used in criminology or forensic science * Partial seizure or focal seizure, a seizure that initially affects only one hemisphere of the brain * Partial or Part score, in contract bridge a trick score less than 100, as well as other meanings * Partial or Partial wave, one sound wave of which a complex tone is com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta Decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino; or, conversely a proton is converted into a neutron by the emission of a positron with a neutrino in what is called ''positron emission''. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by its nuclear binding energy. The binding energies of all existing nuclides form what is called the nuclear band or valley of stability. For either electron or positron emission to be energeticall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Beta Decay
In nuclear physics, double beta decay is a type of radioactive decay in which two neutrons are simultaneously transformed into two protons, or vice versa, inside an atomic nucleus. As in single beta decay, this process allows the atom to move closer to the optimal ratio of protons and neutrons. As a result of this transformation, the nucleus emits two detectable beta particles, which are electrons or positrons. The literature distinguishes between two types of double beta decay: ''ordinary'' double beta decay and ''neutrinoless'' double beta decay. In ordinary double beta decay, which has been observed in several isotopes, two electrons and two electron antineutrinos are emitted from the decaying nucleus. In neutrinoless double beta decay, a hypothesized process that has never been observed, only electrons would be emitted. History The idea of double beta decay was first proposed by Maria Goeppert Mayer in 1935. In 1937, Ettore Majorana demonstrated that all results of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead-206
Lead (82Pb) has four observationally stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains: the uranium series (or radium series), the actinium series, and the thorium series, respectively; a fourth decay chain, the neptunium series, terminates with the thallium isotope 205Tl. The three series terminating in lead represent the decay chain products of long-lived primordial 238U, 235U, and 232Th. Each isotope also occurs, to some extent, as primordial isotopes that were made in supernovae, rather than radiogenically as daughter products. The fixed ratio of lead-204 to the primordial amounts of the other lead isotopes may be used as the baseline to estimate the extra amounts of radiogenic lead present in rocks as a result of decay from uranium and thorium. (See lead–lead dating and uranium–lead dating.) The longest-liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polonium-218
There are 42 isotopes of polonium (84Po). They range in size from 186 to 227 nucleons. They are all radioactive. 210Po with a half-life of 138.376 days has the longest half-life of any naturally-occurring isotope of polonium and is the most common isotope of polonium. It is also the most easily synthesized polonium isotope. 209Po, which does not occur naturally, has the longest half-life of all isotopes of polonium at 124 years. 209Po can be made by using a cyclotron to bombard bismuth with protons, as can 208Po. List of isotopes , -id=Polonium-186 , 186Po , , style="text-align:right" , 84 , style="text-align:right" , 102 , 186.004403(20) , , α , 182Pb , 0+ , , -id=Polonium-187 , 187Po , , style="text-align:right" , 84 , style="text-align:right" , 103 , 187.003030(40) , 1.40(25) ms , α , 183Pb , 1/2−, 5/2− , , -id=Polonium-187m , style="text-indent:1em" , 187mPo , , colspan="3" style="text-indent:2em" , 4(27) keV , 0.5 ms , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of and a mass of , and is represented as ^_\alpha. For example, uranium-238 undergoes alpha decay to form thorium-234. While alpha particles have a charge , this is not usually shown because a nuclear equation describes a nuclear reaction without considering the electrons – a convention that does not imply that the nuclei necessarily occur in neutral atoms. Alpha decay typically occurs in the heaviest nuclides. Theoretically, it can occur only in nuclei somewhat heavier than nickel (element 28), where the overall binding energy per nucleon is no longer a maximum a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |