|
Radafaxine
Radafaxine (developmental code GW-353,162; also known as (2''S'',3''S'')-hydroxybupropion or (''S'',''S'')-hydroxybupropion) is a norepinephrine–dopamine reuptake inhibitor (NDRI) which was under development by GlaxoSmithKline in the 2000s for a variety of different indications but was never marketed. These uses included treatment of restless legs syndrome, major depressive disorder, bipolar disorder, neuropathic pain, fibromyalgia, and obesity. Regulatory filing was planned for 2007, but development was discontinued in 2006 due to "poor test results". Pharmacology Pharmacodynamics Radafaxine is described as a norepinephrine–dopamine reuptake inhibitor (NDRI). In contrast to bupropion, it appears to have a higher potency on inhibition of norepinephrine reuptake than on dopamine reuptake. Radafaxine has about 70% of the efficacy of bupropion in blocking dopamine reuptake, and 392% of efficacy in blocking norepinephrine reuptake, making it fairly selective for inhibiting the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(2R,3R)-Hydroxybupropion
(2''R'',3''R'')-Hydroxybupropion, or simply (''R'',''R'')-hydroxybupropion, is the major metabolite of the antidepressant, smoking cessation, and appetite suppressant medication bupropion. It is the (2''R'',3''R'')-enantiomer of hydroxybupropion, which in humans occurs as a racemic mixture, mixture of (2''R'',3''R'')-hydroxybupropion and radafaxine, (2''S'',3''S'')-hydroxybupropion (radafaxine). Hydroxybupropion is formed from bupropion mainly by the cytochrome P450 enzyme CYP2B6. Levels of (2''R'',3''R'')-hydroxybupropion are dramatically higher than those of bupropion and its other metabolites during bupropion therapy. Exposure with bupropion Bupropion is substantially converted into metabolites during first pass effect, first-pass metabolism with oral administration and levels of its metabolites are much higher than those of bupropion itself. Exposure to (2''R'',3''R'')-hydroxybupropion is 29-fold higher than to (''R'')-bupropion and exposure to (2''S'',3''S'')-hydroxybupropion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bupropion
Bupropion, formerly called amfebutamone, and sold under the brand name Wellbutrin among others, is an atypical antidepressant that is indicated in the treatment of major depressive disorder, seasonal affective disorder, and to support smoking cessation. It is also popular as an add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction, it is not associated with weight gain and sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion, particularly the immediate-release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder. The medication is taken by mouth. Common adverse effects of bupropion with the greatest difference fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylmorpholines
Substituted phenylmorpholines, or substituted phenmetrazines alternatively, are chemical derivatives of 2-phenylmorpholine or of the psychostimulant drug phenmetrazine. Most such compounds act as Monoamine releasing agent, releasers of monoamine neurotransmitters, and have stimulant effects. Some also act as agonists at serotonin receptors, and compounds with an N-propyl substitution act as dopamine receptor agonists. A number of derivatives from this class have been investigated for medical applications, such as for use as anorectics or medications for the treatment of ADHD. Some compounds have also become subject to illicit use as designer drugs. List of phenylmorpholines Additional phenylmorpholines and their activities as monoamine releasing agents (MRAs) have been described. See also * Substituted amphetamine * Substituted benzofuran * Substituted cathinone * Substituted methylenedioxyphenethylamine * List of aminorex analogues * List of methylphenidate analogues * Li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Chlorophenmetrazine
3-Chlorophenmetrazine (3-CPM; code name PAL-594) is a recreational designer drug with stimulant effects. It is a substituted phenylmorpholine derivative, closely related to better known drugs such as phenmetrazine and 3-fluorophenmetrazine (3-FPM; PAL-593). The drug has been shown to act as a norepinephrine–dopamine releasing agent (NDRA) with additional weak serotonin release. Its values for induction of monoamine release are 27nM for dopamine, 75nM for norepinephrine, and 301nM for serotonin in rat brain synaptosomes. Hence, it releases dopamine about 3-fold more potently than norepinephrine and about 11-fold more potently than serotonin. Similarly to ''cis''-4-methylaminorex, the drug is notable in being one of the most selective dopamine releasing agents (DRAs) known, although it still has substantial capacity to release norepinephrine. See also * 3-Bromomethylphenidate * 3-Chloromethamphetamine * 3-Chloromethcathinone * 4-Methylphenmetrazine * G-130 * Methylen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxybupropion
Hydroxybupropion (code name BW 306U), or 6-hydroxybupropion, is the major active metabolite of the antidepressant and smoking cessation drug bupropion. It is formed from bupropion by the liver enzyme CYP2B6 during first-pass metabolism. With oral bupropion treatment, hydroxybupropion is present in blood plasma, plasma at area under the curve (pharmacokinetics), area under the curve concentrations that are as many as 16 to 20 times greater than those of bupropion itself, demonstrating extensive conversion of bupropion into hydroxybupropion in humans. As such, hydroxybupropion is likely to play a very important role in the effects of oral bupropion, which could accurately be thought of as functioning largely as a prodrug to hydroxybupropion. Hydroxybupropion has two chiral centers and is a racemic mixture, mixture of four possible enantiomers. In humans however, presumably due to steric hindrance, only (2R,3R)-Hydroxybupropion, (2''R'',3''R'')-hydroxybupropion and (2S,3S)-Hydroxybup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
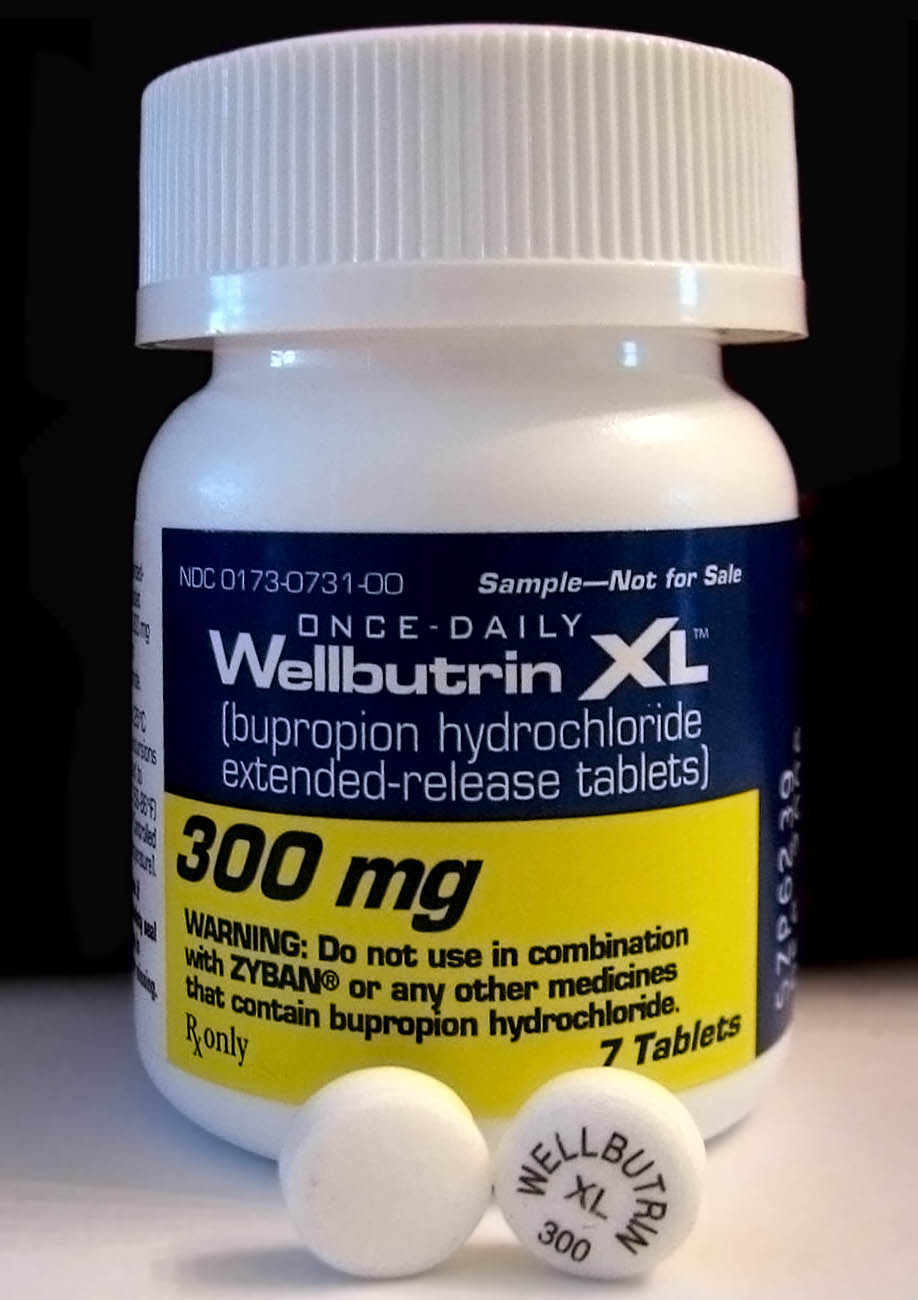
Wellbutrin
Bupropion, formerly called amfebutamone, and sold under the brand name Wellbutrin among others, is an atypical antidepressant that is indicated in the treatment of major depressive disorder, seasonal affective disorder, and to support smoking cessation. It is also popular as an adjunct therapy, add-on medication in the cases of "incomplete response" to the first-line selective serotonin reuptake inhibitor (SSRI) antidepressant. Bupropion has several features that distinguish it from other antidepressants: it does not usually cause sexual dysfunction, it is not associated with weight gain and somnolence, sleepiness, and it is more effective than SSRIs at improving symptoms of hypersomnia and fatigue. Bupropion, particularly the immediate-release formulation, carries a higher risk of seizure than many other antidepressants, hence caution is recommended in patients with a history of seizure disorder. The medication is taken oral administration, by mouth. Common adverse effects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antidepressants
Antidepressants are a class of medications used to treat major depressive disorder, anxiety disorders, chronic pain, and addiction. Common side effects of antidepressants include dry mouth, weight gain, dizziness, headaches, akathisia, sexual dysfunction, and emotional blunting. There is an increased risk of suicidal thinking and behavior when taken by children, adolescents, and young adults. Discontinuation syndrome, which resembles recurrent depression in the case of the SSRI class, may occur after stopping the intake of any antidepressant. Research regarding the effectiveness of antidepressants for depression in adults is controversial and has found both benefits and drawbacks. Meanwhile, evidence of benefit in children and adolescents is unclear, even though antidepressant use has considerably increased in children and adolescents in the 2000s. While a 2018 study found that the 21 most commonly prescribed antidepressant medications were slightly more effective than p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abandoned Drugs
Abandon, abandoned, or abandonment may refer to: Common uses * Abandonment (emotional), a subjective emotional state in which people feel undesired, left behind, insecure, or discarded * Abandonment (legal), a legal term regarding property ** Child abandonment, the extralegal abandonment of children ** Lost, mislaid, and abandoned property, legal status of property after abandonment and rediscovery * Abandonment (mysticism) Art, entertainment, and media Film * ''Abandon'' (film), a 2002 film starring Katie Holmes * ''Abandoned'' (1949 film), starring Dennis O'Keefe * ''Abandoned'' (1955 film), the English language title of the Italian war film ''Gli Sbandati'' * ''Abandoned'' (2001 film), a Hungarian film * ''Abandoned'' (2010 film), starring Brittany Murphy * ''Abandoned'' (2015 film), a television movie about the shipwreck of the ''Rose-Noëlle'' in 1989 * ''Abandoned'' (2022 film), starring Emma Roberts * ''The Abandoned'' (1945 film), a 1945 Mexican film * ''The Aba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |