|
Producer Gas
Producer gas is a fuel gas manufactured by blowing air and steam simultaneously through a coke or coal fire. It mainly consists of carbon monoxide (CO), hydrogen (H2), as well as substantial amounts of nitrogen (N2). The caloric value of the producer gas is low (mainly because of its high nitrogen content), and the technology is obsolete. Improvements over producer gas, also obsolete, include water gas, where the solid fuel is treated intermittently with air and steam, and, far more efficiently, synthesis gas, where the solid fuel is replaced with methane. In the US, producer gas may also be referred to by other names based on the fuel used for production, such as wood gas. Producer gas may also be referred to as suction gas, referring to the way the air was drawn into the gas generator by an internal combustion engine. Production Producer gas is generally made from coke, or other carbonaceous material such as anthracite coal. Air is passed over the red-hot carbonaceous fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adler Diplomat 3 GS Mit Holzgasgenerator-hinten Rechts
Adler may refer to: Places *Adler, Alabama, an unincorporated community in Perry County *Adler Planetarium, Chicago, Illinois, USA *Adler Township, Nelson County, North Dakota, USA *Adler University, formerly Adler School of Professional Psychology, in Chicago, Illinois, USA * Adlersky City District, Sochi, Russia **Adler Microdistrict, a resort in Sochi, Russia **Adler railway station, a station serving the city Sports *Adler Mannheim, a German ice hockey team *Berlin Adler, an American football team in Berlin *Nickname of the sports club Eintracht Frankfurt *Nickname for the Germany national football team Transportation *, a number of steamships *Adler (cars and motorcycle), an early 20th-century automobile. The firm also produced typewriters and other office equipment. *Adler (locomotive), the first German steam locomotive (1835) *Adler or Adlerwerke vorm. Heinrich Kleyer, a German aircraft manufacturer Other uses *Adler (band), an American rock band *Adler (comics), a Fra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calorific Value
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it. The ''calorific value'' is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon or other organic molecule reacting with oxygen to form carbon dioxide and water and release heat. It may be expressed with the quantities: * energy/ mole of fuel * energy/mass of fuel * energy/volume of the fuel There are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much the products are allowed to cool and whether compounds like are allowed to condense. The high heat values are conventionally measured with a bomb calorimeter. Low heat values are calculated from high heat value test data. They may also be calculated as the difference betwe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Gas
Water gas is a kind of fuel gas, a mixture of carbon monoxide and hydrogen. It is produced by "alternately hot blowing a fuel layer okewith air and gasifying it with steam". The caloric yield of the fuel produced by this method is about 10% of the yield from a modern syngas plant. The coke needed to produce water gas also costs significantly more than the precursors for syngas (mainly methane from natural gas), making water gas technology an even less attractive business proposition. Production Synthesis gas is made by passing steam over a red-hot carbon fuel such as coke: : (Δ''H'' = +131 kJ/mol) The reaction is endothermic, so the fuel must be continually re-heated to maintain the reaction. To do this, an air stream, which alternates with the vapor stream, is introduced to combust some of the carbon: : (Δ''H'' = −393 kJ/mol) Theoretically, to make 6 L of water gas, 5 L of air is required. Alternatively, to prevent contamination with nitrogen, energy can be p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology The word ''pyrolysis'' is coined from the Greek language, Greek-derived morpheme, elements ''pyro-'' (from Ancient Greek : - "fire, heat, fever") and ''lysis'' ( : - "separation, loosening"). Applications Pyrolysis is most commonly used in the treatment of organic compound, organic materials. It is one of the processes involved in the charring of wood or pyrolysis of biomass. In general, pyrolysis of organic substances produces volatile products and leaves Char (chemistry), char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered one of the steps in the processes of gasification or combustion. Laypeople often confuse pyrolysis gas with syngas. Pyrolysis gas has a high percentage of heavy tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
History Of Manufactured Gas
The history of gaseous fuel, important for lighting, heating, and cooking purposes throughout most of the 19th century and the first half of the 20th century, began with the development of analytical and pneumatic chemistry in the 18th century. These "synthetic fuel gases" (also known as "manufactured fuel gas", "manufactured gas" or simply "gas") were made by gasification of combustible materials, usually coal, but also wood and oil, by heating them in enclosed ovens with an oxygen-poor atmosphere. The fuel gases generated were mixtures of many chemical substances, including hydrogen, methane, carbon monoxide and ethylene. Coal gas also contains significant quantities of unwanted sulfur and ammonia compounds, as well as heavy hydrocarbons, and must be purified before use. The first attempts to manufacture fuel gas in a commercial way were made in the period 1795–1805 in France by Philippe LeBon, and in England by William Murdoch. Although precursors can be found, it was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasifier
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock. An advantage of gasification is that syngas can be more efficient than direct combustion of the original feedstock material because it can be combusted at higher temperatures so that the thermodynamic upper limit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gasification
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock. An advantage of gasification is that syngas can be more efficient than direct combustion of the original feedstock material because it can be combusted at higher temperatures so that the thermodynamic upper limi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fuel Gas
Fuel gas is one of a number of fuels that under ordinary conditions are gaseous. Most fuel gases are composed of hydrocarbons (such as methane and propane), hydrogen, carbon monoxide, or mixtures thereof. Such gases are sources of energy that can be readily transmitted and distributed through pipes. Fuel gas is contrasted with liquid fuels and solid fuels, although some fuel gases are Liquefaction of gases, liquefied for storage or transport (for example, autogas and Liquefied petroleum gas, liquified petroleum gas). While their gaseous nature has advantages, avoiding the difficulty of transporting solid fuel and the dangers of spillage inherent in liquid fuels, it also has limitations. It is possible for a fuel gas to be undetected and cause a gas explosion. For this reason, odorizers are added to most fuel gases. The most common type of fuel gas in current use is natural gas. Types of fuel gas There are two broad classes of fuel gases, based not on their chemical composition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
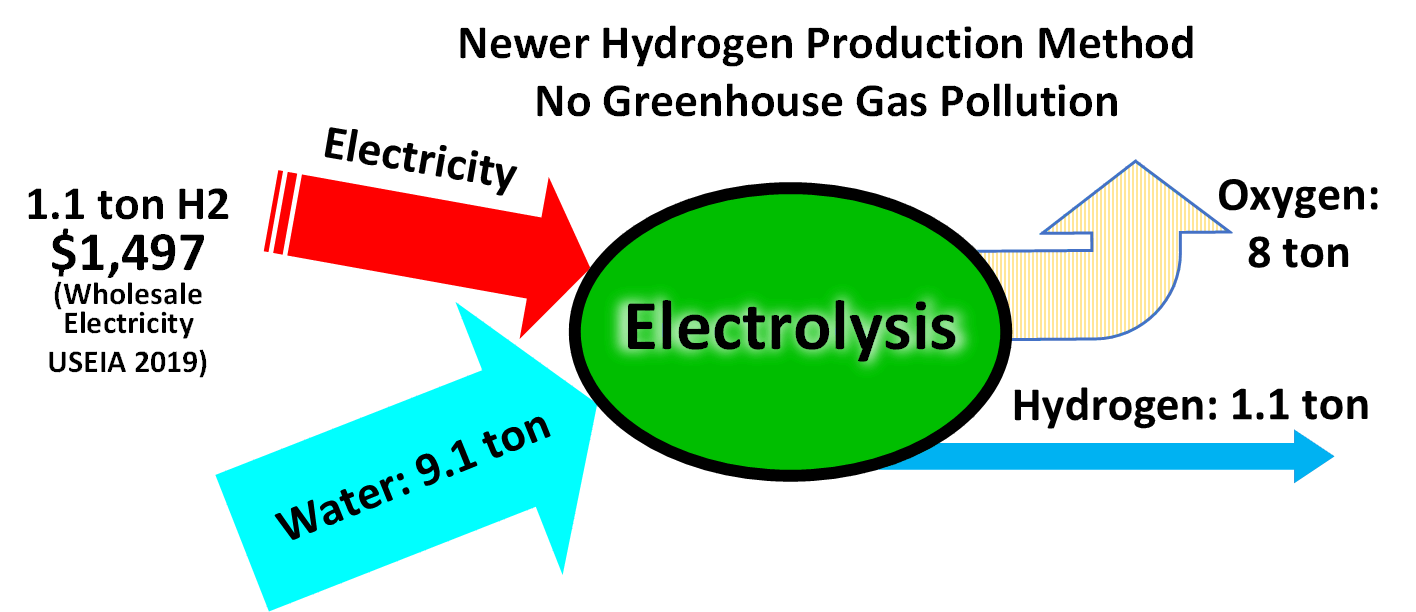
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Syngas
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII (in Germany alone, half a million cars were built or rebuilt to run on wood gas). Production Syngas is produced by steam reforming or partial oxidation of natural gas or liquid hydrocarbons, or coal gasification. : : : Steam reforming of methane is an endothermic reaction requiring 206 kJ/mol of energy: : In principle, but rarely in practice, biomass and related hydrocarbon feedstocks could be used to generate biogas and biochar in waste-to-energy gasification facilities. The gas generated (mostly methane and carbon dioxide) is sometimes described as ''syng ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Mantle
A Coleman white gas lantern mantle glowing at full brightness An incandescent gas mantle, gas mantle or Welsbach mantle is a device for generating bright white light when heated by a flame. The name refers to its original heat source in gas lights which illuminated the streets of Europe and North America in the late 19th century. ''Mantle'' refers to the way it hangs like a cloak above the flame. Gas mantles are also used in some portable camping lanterns, pressure lanterns and some oil lamps. Gas mantles are usually sold as a fabric bag which, because of impregnation with metal nitrates, burns away to leave a rigid but fragile mesh of metal oxides when heated during initial use; these metal oxides produce light from the heat of the flame whenever used. Thorium dioxide was commonly a major component; being radioactive, it has led to concerns about the safety of those involved in manufacturing mantles. Normal use, however, poses minimal health risk. Mechanism left, Hot g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercaptan
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of Alcohol (chemistry), alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). becaus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |