|
Potassium Cycle
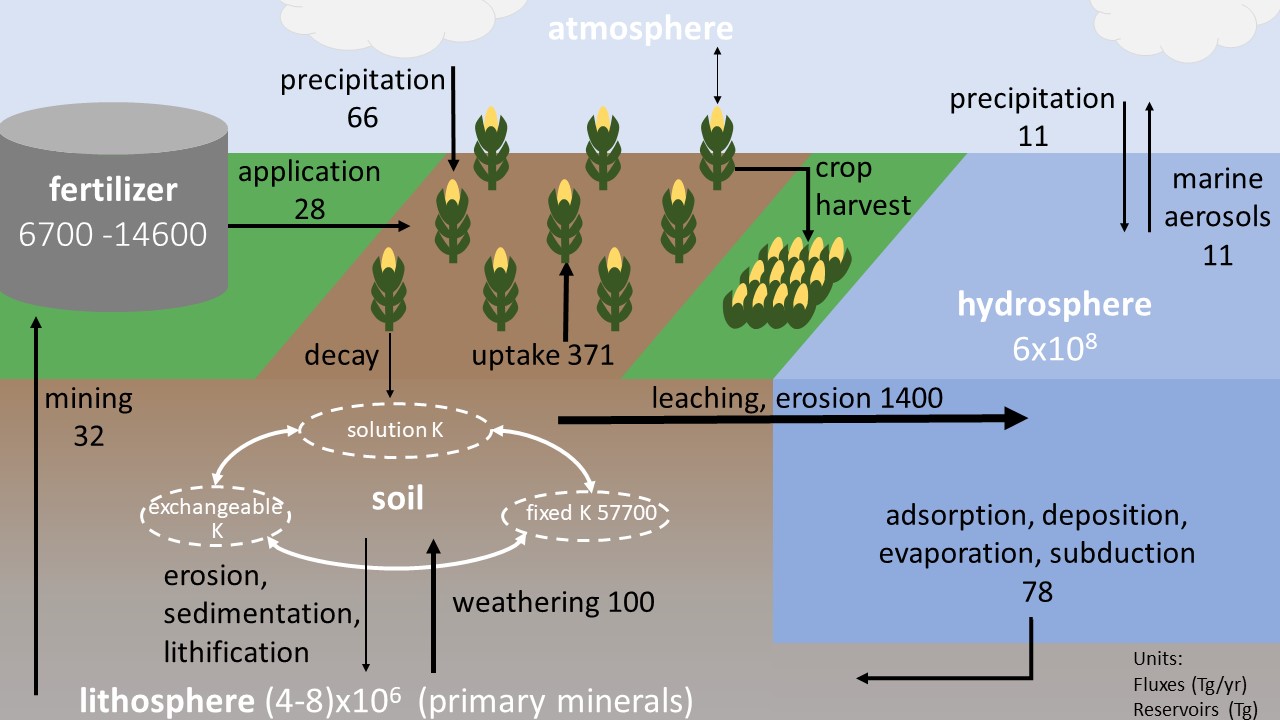
The potassium (K) cycle is the biogeochemical cycle that describes the movement of potassium throughout the Earth’s lithosphere, biosphere, atmosphere, and hydrosphere. Functions Along with nitrogen and phosphorus, potassium is one of the three major nutrients that plants require in large quantities. Potassium is essential to stomata control in plants and is also essential for muscles contraction in humans. Lithosphere and Soil By weight, K totals to 2.6% of the Earth’s crust. Stored in primary minerals (feldspar, biotite, and muscovite), chemical weathering releases potassium into the soil to account for up to 11% of plant demand. Some plants and bacteria also release organic acids into the soil that make K accessible for their use. Potassium exists in its highest concentrations in the upper most layers of soil, stored in three pools: fixed K, exchangeable K, and solution K. Fixed K accounts for 96-99% of soil K and is stored in feldspar, mica, and illite minerals. Exc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
K Cycle Figure Draft 5
K, or k, is the eleventh letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''kay'' (pronounced ), plural ''kays''. The letter K usually represents the voiceless velar plosive. History The letter K comes from the Greek letter Κ (kappa), which was taken from the Semitic kaph, the symbol for an open hand. This, in turn, was likely adapted by Semitic tribes who had lived in Egypt from the hieroglyph for "hand" representing /ḏ/ in the Egyptian word for hand, ⟨ ḏ-r-t⟩ (likely pronounced in Old Egyptian). The Semites evidently assigned it the sound value instead, because their word for hand started with that sound. K was brought into the Latin alphabet with the name ''ka'' /kaː/ to differentiate it from C, named ''ce'' (pronounced /keː/) and Q, named ''qu'' and pronounced /kuː/. In the earliest Latin inscriptions, the letters C, K and Q were all used to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a '' surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term '' sorption'' encompasses both processes, while '' desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potash
Potash () includes various mined and manufactured salts that contain potassium in water- soluble form.Potash USGS 2008 Minerals Yearbook The name derives from ''pot ash'', plant ashes or wood ash soaked in water in a pot, the primary means of manufacturing potash before the Industrial Era. The word '''' is derived from ''potash''. Potash is produced worldwide in amounts exceeding 90 million s (40 mil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subduction
Subduction is a geological process in which the oceanic lithosphere is recycled into the Earth's mantle at convergent boundaries. Where the oceanic lithosphere of a tectonic plate converges with the less dense lithosphere of a second plate, the heavier plate dives beneath the second plate and sinks into the mantle. A region where this process occurs is known as a subduction zone, and its surface expression is known as an arc-trench complex. The process of subduction has created most of the Earth's continental crust. Rates of subduction are typically measured in centimeters per year, with the average rate of convergence being approximately two to eight centimeters per year along most plate boundaries. Subduction is possible because the cold oceanic lithosphere is slightly denser than the underlying asthenosphere, the hot, ductile layer in the upper mantle underlying the cold, rigid lithosphere. Once initiated, stable subduction is driven mostly by the negative buoyancy of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abscission
Abscission () is the shedding of various parts of an organism, such as a plant dropping a leaf, fruit, flower, or seed. In zoology, abscission is the intentional shedding of a body part, such as the shedding of a claw, husk, or the autotomy of a tail to evade a predator. In mycology, it is the liberation of a fungal spore. In cell biology, abscission refers to the separation of two daughter cells at the completion of cytokinesis. In plants Function A plant will abscise a part either to discard a member that is no longer necessary, such as a leaf during autumn, or a flower following fertilisation, or for the purposes of reproduction. Most deciduous plants drop their leaves by abscission before winter, whereas evergreen plants continuously abscise their leaves. Another form of abscission is fruit drop, when a plant abscises fruit while still immature, in order to conserve resources needed to bring the remaining fruit to maturity. If a leaf is damaged, a plant may also abscise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaching (agriculture)
In agriculture, leaching is the loss of water-soluble plant nutrients from the soil, due to rain and irrigation. Soil structure, crop planting, type and application rates of fertilizers, and other factors are taken into account to avoid excessive nutrient loss. Leaching may also refer to the practice of applying a small amount of excess irrigation where the water has a high salt content to avoid salts from building up in the soil ( salinity control). Where this is practiced, drainage must also usually be employed, to carry away the excess water. Leaching is a natural environment concern when it contributes to groundwater contamination. As water from rain, flooding, or other sources seeps into the ground, it can dissolve chemicals and carry them into the underground water supply. Of particular concern are hazardous waste dumps and landfills, and, in agriculture, excess fertilizer, improperly stored animal manure, and biocides (e.g. pesticides, fungicides, insecticides and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : K2O2 + 2 K -> 2 K2O Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: :2KNO3 + 10K -> 6K2O + N2 (^) Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. :2K2O2 -> 2K2O + O2 (^) Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potash
Potash () includes various mined and manufactured salts that contain potassium in water- soluble form.Potash USGS 2008 Minerals Yearbook The name derives from ''pot ash'', plant ashes or wood ash soaked in water in a pot, the primary means of manufacturing potash before the Industrial Era. The word '''' is derived from ''potash''. Potash is produced worldwide in amounts exceeding 90 million s (40 mil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners (as a substitute for sodium chloride salt), and in food processing, where it may be known as E number additive E508. It occurs naturally as the mineral sylvite, and in combination with sodium chloride as sylvinite. Uses Fertilizer The majority of the potassium chloride produced is used for making fertilizer, called potash, since the growth of many plants is limited by potassium availability. Potassium chloride sold as fertilizer is known as muriate of potash (MOP). The vast majority of potash fertilizer worldwide is sold as MOP. Medical use Potassium is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporite
An evaporite () is a water- soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocean deposits, and non-marine, which are found in standing bodies of water such as lakes. Evaporites are considered sedimentary rocks and are formed by chemical sediments. Formation of evaporite rocks Although all water bodies on the surface and in aquifers contain dissolved salts, the water must evaporate into the atmosphere for the minerals to precipitate. For this to happen, the water body must enter a restricted environment where water input into this environment remains below the net rate of evaporation. This is usually an arid environment with a small basin fed by a limited input of water. When evaporation occurs, the remaining water is enriched in salts, and they precipitate when the water becomes supersaturated. Evaporite depo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |