|
Potassium Chromate
Potassium chromate is the inorganic compound with the formula Potassium, K2Chromate ion, CrO4. This yellow solid is the potassium salt of the Chromate ion, chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially. Structure Two crystalline forms are known, both being very similar to the corresponding potassium sulfate. Orthorhombic β-K2CrO4 is the common form, but it converts to an α-form above 666 °C.Gerd Anger, Jost Halstenberg, Klaus Hochgeschwender, Christoph Scherhag, Ulrich Korallus, Herbert Knopf, Peter Schmidt, Manfred Ohlinger, "Chromium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. These structures are complex, although the chromate ion adopts the typical tetrahedral geometry.Gaultier, M.; Pannetier, G. "Structure cristalline de la forme 'basse temperature' du sulfate de potassium K2SO4-beta" (Crystal structure of the "low temperature" β-form of potassium sulfate) Bulletin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium Trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula . It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen. Production, structure, and basic reactions Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: : Approximately 100,000 tonnes are produced annually by this or similar routes. The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3. The structure of monomeric has been ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Compounds
Potassium is a chemical element; it has symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a common constituent of granites and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosive
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen, or hydroxide. Rusting, the formation of red-orange iron oxides, is a well-known example of electrochemical corrosion. This type of corrosion typically produces oxides or salts of the original metal and results in a distinctive coloration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including mechanical strength, appearance, and permeability to liquids and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent which relate to the agent's ability to engage dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cr(VI)
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is any chemical compound that contains the element chromium in the +6 oxidation state (thus hexavalent). It has been identified as carcinogenic, which is of concern since approximately of hexavalent chromium were produced in 1985. Hexavalent chromium compounds can be carcinogens ( IARC Group 1), especially if airborne and inhaled where they can cause lung cancer. Occurrence and uses Hexavalent chromium occurs only rarely in nature, an exception being crocoite (PbCrO4). It is however produced on a large scale industrially. Virtually all chromium ore is processed via the formation of hexavalent chromium, specifically the salt sodium dichromate. Sodium chromate is converted into other hexavalent chromium compounds such as chromium trioxide and various salts of chromate and dichromate. Industrial uses of hexavalent chromium compounds include chromate pigments in dyes, paints, inks, and plastics; chromates added as anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atacama Desert
The Atacama Desert () is a desert plateau located on the Pacific Ocean, Pacific coast of South America, in the north of Chile. Stretching over a strip of land west of the Andes Mountains, it covers an area of , which increases to if the barren lower slopes of the Andes are included. The Atacama Desert is the driest nonpolar desert in the world, and the second driest overall, behind some specific spots within the McMurdo Dry Valleys. It is the only Desert climate, true desert to receive less precipitation than polar deserts, and the largest fog desert in the world. The area has been used as an experimentation site for Mars expedition simulations due to its similarities to the Martian environment. The constant Inversion (meteorology), temperature inversion caused by the cool north-flowing Humboldt Current, Humboldt ocean current and the strong South Pacific High, Pacific anticyclone contribute to the extreme aridity of the desert. The most arid region of the Atacama Desert is s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tarapacaite
Tarapacáite is the mineral form of potassium chromate with the chemical formula K2 Cr O4. It forms bright yellow crystals and was discovered in 1878. It is named for the former Tarapacá Province, Peru; nowadays belonging to Chile. The boundaries between Peru, Bolivia and Chile were vague in the Atacama Desert before the War of the Pacific (1879–1883). Its type locality is Oficina Maria Elena, Maria Elena, Tocopilla Province Tocopilla Province () is one of the three provinces in the northern Chilean region of Antofagasta (II). Its capital is the city of Tocopilla. Geography and demography According to the 2012 census by the National Statistics Institute (''INE''), t ..., Antofagasta Region, Chile. It is unlikely to occur anywhere except in highly arid conditions as it is easily soluble in water. References Potassium minerals Chromate minerals Orthorhombic minerals Minerals in space group 62 Minerals described in 1878 {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
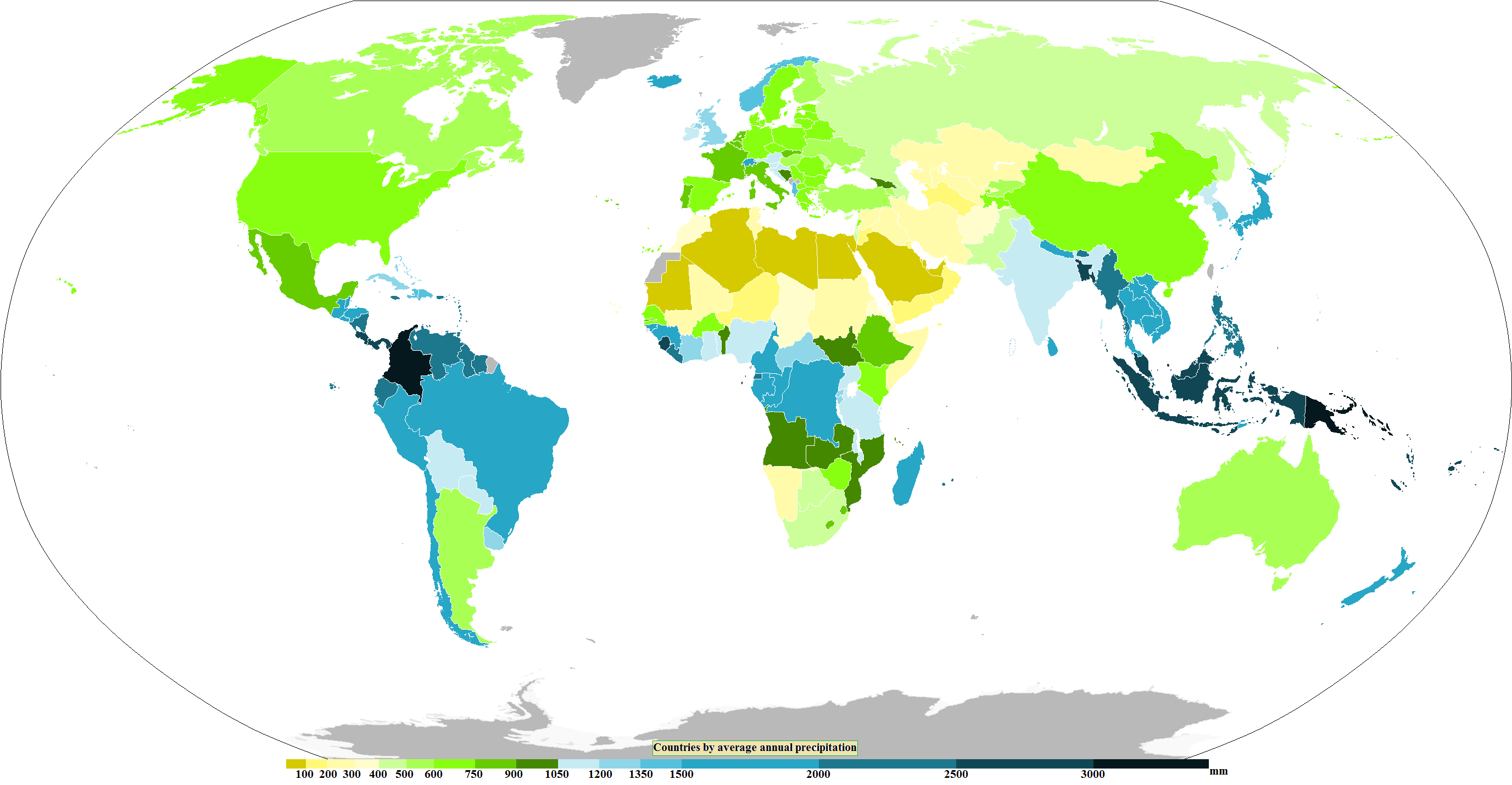
Precipitation Titration
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwealth usage), snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. (Such a non-precipitating combination is a colloid.) Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are called shower (p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualitative Inorganic Analysis
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes. Qualitative inorganic analysis is that branch or method of analytical chemistry which seeks to establish the elemental composition of inorganic compounds through various reagents. Physical appearance of some inorganic compounds Detecting cations According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution. To obtain meaningful results, the separation must be done in the sequence specified bel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, Enantioselective synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject. Total synthesis A total synthesis refers to the complete chemical synthesis of molecules from simple, Precursor (chemistry), natural precursors. Total synthesis is accomplished either via a linear or convergent approach. In a Linear synthesis, ''linear'' synthesis—often adequate for simple structures—several steps are performed sequentially until the molecule is com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidizing Agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens. In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction in which it gains one or more electrons. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |