|
Polydimethylsiloxane
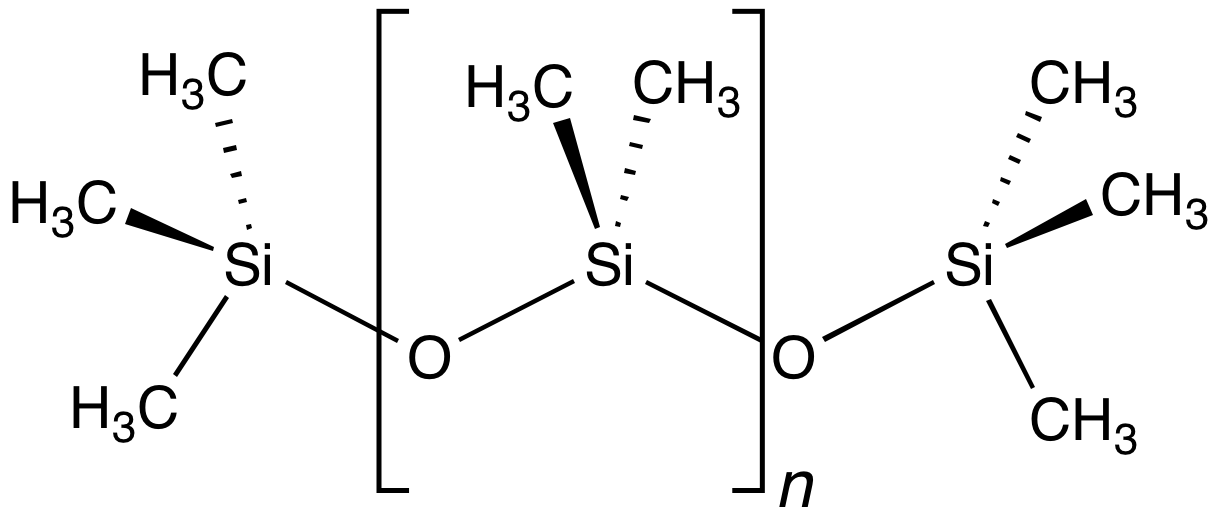
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling. PDMS is particularly known for its unusual rheological (or flow) properties. It is optically clear and, in general, inert, non-toxic, and non-flammable. It is one of several types of silicone oil (polymerized siloxane). The applications of PDMS range from contact lenses and medical devices to elastomers; it is also present in shampoos (as it makes hair shiny and slippery), food ( antifoaming agent), caulk, lubricants and heat-resistant tiles. Structure The chemical formula of PDMS is , where ''n'' is the number of repeating monomer units. Industrial synthesis can begin from dimethyldichlorosilane and water by the following net reaction: : + (''n''+1) The polymerization reaction evolves hydrochloric acid. For medical and domestic applications, a process wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, silicone grease, grease, silicone rubber, rubber, silicone resin, resin, and Caulking, caulk. Silicone is often confused with one of its constituent elements, silicon, but they are distinct substances. Silicon is a chemical element, a hard dark-grey semiconductor, semiconducting metalloid, which in its crystalline form is used to make integrated circuits ("electronic chips") and solar cells. Silicones are compounds that contain silicon, carbon, hydrogen, oxygen, and perhaps other kinds of atoms as well, and have many very different physical and chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antifoaming Agent
A defoamer or an anti-foaming agent is a chemical additive that reduces and hinders the formation of foam in industrial process liquids. The terms anti-foam agent and defoamer are often used interchangeably. Strictly speaking, defoamers eliminate existing foam and anti-foamers prevent the formation of further foam. Commonly used agents are insoluble oils, polydimethylsiloxanes and other silicones, certain alcohols, stearates and glycols. The additive is used to prevent formation of foam or is added to break a foam already formed. In industrial processes, foams pose serious problems. They cause defects on surface coatings and prevent the efficient filling of containers. A variety of chemical formulae are available to prevent formation of foams. Properties Generally a defoamer is insoluble in the foaming medium and has surface active properties. An essential feature of a defoamer product is a low viscosity and a facility to spread rapidly on foamy surfaces. It has affinity to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passive Daytime Radiative Cooling
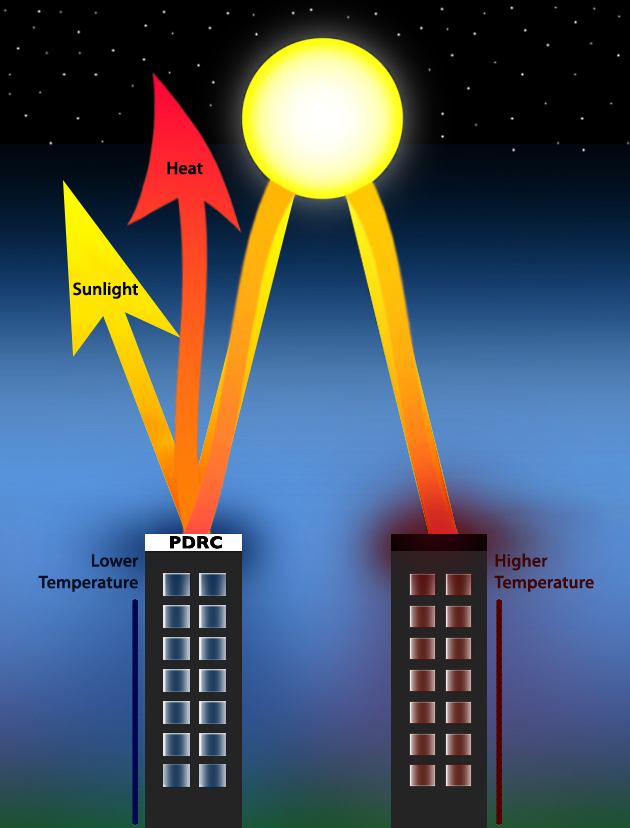
Passive daytime radiative cooling (PDRC) (also passive radiative cooling, daytime passive radiative cooling, radiative sky cooling, photonic radiative cooling, and terrestrial radiative cooling) is the use of unpowered, reflective/Emissivity, thermally-emissive surfaces to lower the temperature of a building or other object. It has been proposed as a method of reducing temperature increases caused by greenhouse gases by reducing the energy needed for air conditioning, lowering the Urban heat island, urban heat island effect, and lowering human body temperatures. PDRCs can aid systems that are more efficient at lower temperatures, such as photovoltaic systems, dew collection devices, and thermoelectric generators. Some estimates propose that dedicating 1–2% of the Earth's surface area to PDRC would stabilize surface temperatures. Regional variations provide different cooling potentials with Desert climate, desert and temperate climates benefiting more than tropical climates, att ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siloxane
In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: . The parent siloxanes include the oligomeric and polymeric hydrides with the formulae and . Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones , the premier example of which is polydimethylsiloxane (PDMS). The functional group (where the three Rs may be different) is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility. Structure Siloxanes generally adopt structures expected for linked tetrahedral ("''sp''3-like") centers. The Si−O bond length is 1.64 Å (vs Si–C distance of 1.92 Å) and the Si-O-Si angle is rather open at 142.5°. By ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tiles
Tiles are usually thin, square or rectangular coverings manufactured from hard-wearing material such as ceramic, stone, metal, baked clay, or even glass. They are generally fixed in place in an array to cover roofs, floors, walls, edges, or other objects such as tabletops. Alternatively, tile can sometimes refer to similar units made from lightweight materials such as perlite, wood, and mineral wool, typically used for wall and ceiling applications. In another sense, a tile is a construction tile or similar object, such as rectangular counters used in playing games (see tile-based game). The word is derived from the French word ''tuile'', which is, in turn, from the Latin word ''tegula'', meaning a roof tile composed of fired clay. Tiles are often used to form wall and floor coverings, and can range from simple square tiles to complex or mosaics. Tiles are most often made of ceramic, typically glazed for internal uses and unglazed for roofing, but other materials are also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caulk
Caulk (also known as caulking and calking) is a material used to Seal (mechanical), seal Joint (building), joints or seams against leakage in various structures and piping. The oldest form of caulk consisted of fibrous materials driven into the wedge-shaped seams between boards on Boat building#Wood, wooden boats or ships. Cast iron Sanitary sewer, sewerage Water pipe, pipes were formerly caulked in a similar way. Riveted seams in ships and boilers were formerly sealed by hammering the metal. Modern caulking compounds are flexible sealing compounds used to close up gaps in buildings and other structures against water, air, dust, insects, or as a component in firestopping. In the tunneling industry, caulking is the sealing of joints in segmental precast concrete tunnels, commonly by using concrete. Historical uses Wooden shipbuilding Traditional caulking (also spelled calking) on wooden vessels uses fibers of cotton and oakum (hemp) soaked in pine tar. These fibers are drive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lubricant
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, transporting foreign particles, or heating or cooling the surfaces. The property of reducing friction is known as lubricity. In addition to industrial applications, lubricants are used for many other purposes. Other uses include cooking (oils and fats in use in frying pans and baking to prevent food sticking), to reduce rusting and friction in machinery, through the use of motor oil and Grease (lubricant), grease, bioapplications on humans (e.g., lubricants for Replacement joint, artificial joints), ultrasound examination, medical examination, and sexual intercourse. It is mainly used to reduce friction and to contribute to a better, more efficient functioning of a mechanism. History Lubricants have been in some use for thousands of year ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Resistance
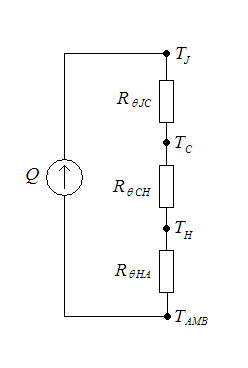
In heat transfer, thermal engineering, and thermodynamics, thermal conductance and thermal resistance are fundamental concepts that describe the ability of materials or systems to conduct heat and the opposition they offer to the heat current. The ability to manipulate these properties allows engineers to control temperature gradient, prevent thermal shock, and maximize the efficiency of thermal systems. Furthermore, these principles find applications in a multitude of fields, including materials science, mechanical engineering, electronics, and energy management. Knowledge of these principles is crucial in various scientific, engineering, and everyday applications, from designing efficient temperature control, thermal insulation, and thermal management in industrial processes to optimizing the performance of electronic devices. Thermal conductance (''G'') measures the ability of a material or system to conduct heat. It provides insights into the ease with which heat can pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Transition
The glass–liquid transition, or glass transition, is the gradual and Reversible reaction, reversible transition in amorphous solid, amorphous materials (or in amorphous regions within Crystallinity, semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. International Organization for Standardization, ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting point, melting temperature, ''T''m, of the cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slippery
Slipperiness is when a surface has a low coefficient of friction, allowing objects to glide across the surface. People walking on slippery surfaces are likely to slip or fall. A surface can for example be slippery due to it being wet, or due to it being icy. There are several competing theories about why ice is slippery. Road slipperiness is a major area of road safety, but various means have also been developed to measure walkway and deck slipperiness in order to develop health and safety standards.Wen-Ruey Chang, Theodore K. Courtney, Raoul Grongvist ''Measuring Slipperiness: Human Locomotion and Surface Factors'' 2002 0415298288 "A number of 'purely' subjective approaches (e.g. paired comparisons) have been applied to measure slipperiness. Human subjects seem to be capable of differentiating the slipperiness of floors (Yoshioka et al. 1978, 1979, Swensen et al." See also *Floor slip resistance testing *Ice cleats Ice cleats are a device, affixed to a shoe or boot, wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyldichlorosilane
Dimethyldichlorosilane is a tetrahedral organosilicon compound with the formula . At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds. History The first organosilicon compounds were reported in 1863 by Charles Friedel and James Crafts who synthesized tetraethylsilane from diethylzinc and silicon tetrachloride.Silicon: Organosilicon Chemistry. Encyclopedia of Inorganic Chemistry Online, 2nd ed.; Wiley: New Jersey, 2005. However, major progress in organosilicon chemistry did not occur until Frederick Kipping and his students began experimenting with diorganodichlorosilanes () that were prepared by reacting silicon tetrachloride with Grignard reagents. Unfortunately, this method suffered from many experimental problems. In the 1930s, the demand for silicones increased due to the need f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |