|
Poloxamer
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene (poly(propylene oxide)) flanked by two hydrophilic chains of polyoxyethylene (poly(ethylene oxide)). The word was coined by BASF inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Pluronic, Kolliphor (pharma grade), and Synperonic. Because the lengths of the polymer blocks can be customized, many different poloxamers exist that have slightly different properties. For the generic term ''poloxamer'', these copolymers are commonly named with the letter ''P'' (for poloxamer) followed by three digits: the first two digits multiplied by 100 give the approximate molecular mass of the polyoxypropylene core, and the last digit multiplied by 10 gives the percentage polyoxyethylene content (e.g. P407 = poloxamer with a polyoxypropylene molecular mass of 4000 g/mol and a 70% polyoxyethylene content). For the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Poloxamer 407
Poloxamer 407 is a hydrophilic non-ionic surfactant of the more general class of copolymers known as poloxamers. Poloxamer 407 is a triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol (PEG). The approximate lengths of the two PEG blocks is 101 repeat units, while the approximate length of the propylene glycol block is 56 repeat units. This particular compound is also known by the BASF trade name Pluronic F-127 or by the Croda trade name Synperonic PE/F 127. BASF also offers a pharmaceutical grade, under trade name Kolliphor P 407. Uses Most of the common uses of poloxamer 407 are related to its surfactant properties. For example, it is widely used in cosmetics for dissolving oily ingredients in water. It can also be found in multi-purpose contact lens cleaning solutions, where its purpose there is to help remove lipid films from the lens. It can also be found in some mouthwashes. There is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Surfactant
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a Blend word, blend of "surface-active agent", coined in 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt. Surfactants are among the most widespread and commercially important chemicals. Private households as well as many industries use them in large quantities as detergent, detergents and cleaning agents, but also for example as emulsion#Emulsifiers, emulsifiers, wetting agents, foaming agents, Antistatic agent, antistatic additives, or dispersants. Surfactants occur naturally in traditional plant-based detergents, e.g. Aesculus, horse chestnuts or Sapindus, soap nuts; they can also be found in the secretions of some caterpillars. Today one of the most commonly used anionic surfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Sonication
image:Sonicator.jpg, A sonicator at the Weizmann Institute of Science during sonicationSonication is the act of applying sound energy to agitate particles in a sample, for various purposes such as the extraction of multiple compounds from plants, microalgae and seaweeds. ultrasound, Ultrasonic frequencies (> 20 kHz) are usually used, leading to the process also being known as ultrasonication or ultra-sonication. In the laboratory, it is usually applied using an ''ultrasonic bath'' or an ''ultrasonic probe'', colloquially known as a ''sonicator''. In a Fourdrinier machine, paper machine, an Ultrasonic foil (papermaking), ultrasonic foil can distribute Cellulose fiber, cellulose fibres more uniformly and strengthen the paper. Effects Sonication has numerous effects, both chemical and physical. The scientific field concerned with understanding the effect of sonic waves on chemical systems is called sonochemistry. The chemical effects of ultrasound do not come from a direc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Micelle
A micelle () or micella () ( or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system). A typical micelle in water forms an aggregate, with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the packing behavior of single-tail lipids in a bilayer. The difficulty in filling the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (or oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (or water-in-oil micelle). Micelles are approximately spherical in shape. Other shapes, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
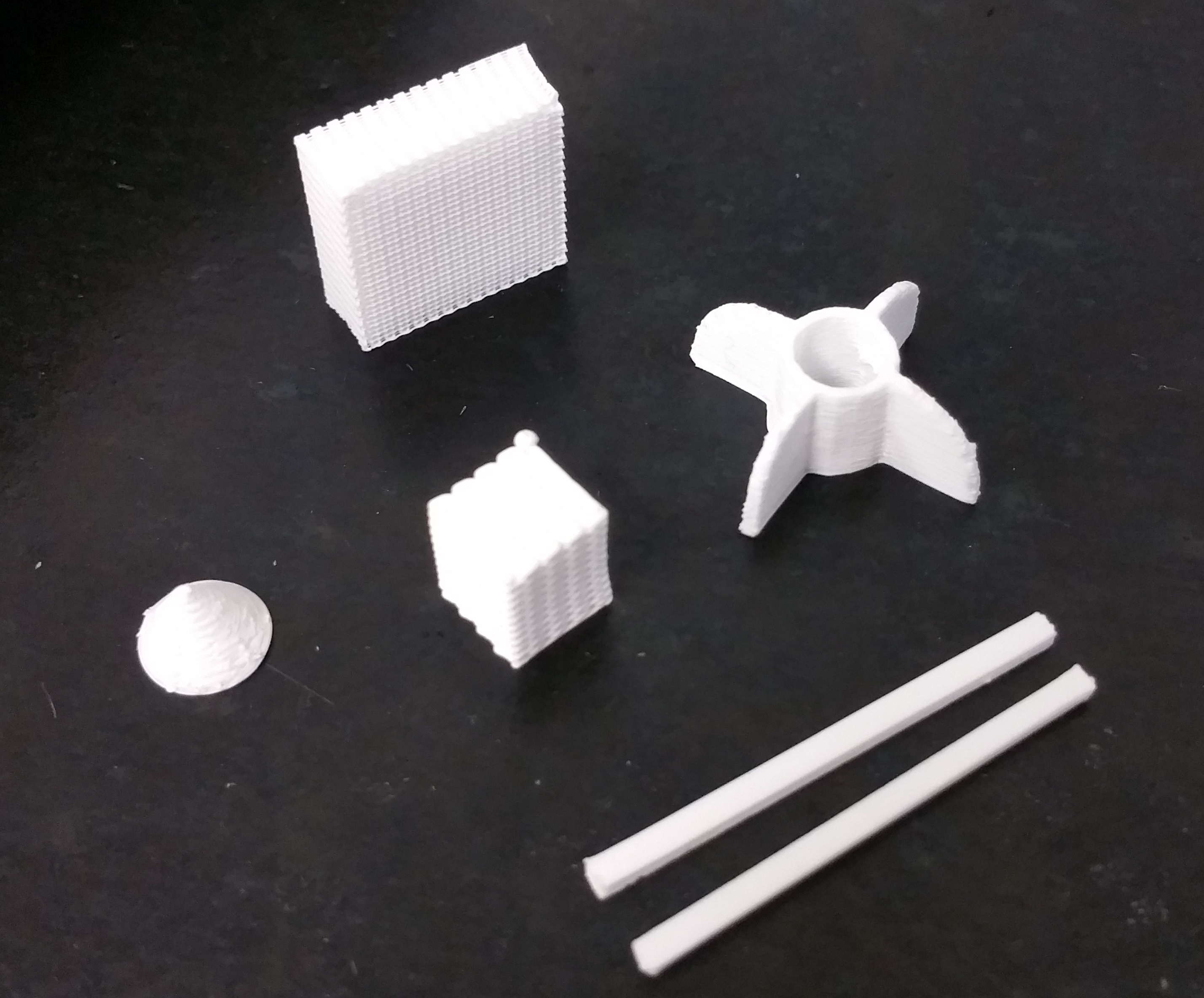
Robocasting
Robocasting (also known as robotic material extrusion) is an additive manufacturing technique analogous to Direct Ink Writing and other extrusion-based 3D-printing techniques in which a filament of a paste-like material is extruded from a small nozzle while the nozzle is moved across a platform. The object is thus built by printing the required shape layer by layer. The technique was first developed in the United States in 1996 as a method to allow geometrically complex ceramic green bodies to be produced by additive manufacturing. In robocasting, a 3D CAD model is divided up into layers in a similar manner to other additive manufacturing techniques. The material (typically a ceramic slurry) is then extruded through a small nozzle as the nozzle's position is controlled, drawing out the shape of each layer of the CAD model. The material exits the nozzle in a liquid-like state but retains its shape immediately, exploiting the rheological property of shear thinning. It is distinct from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Coalescence (physics)
Coalescence is the process by which two or more droplets, bubbles, or particles merge during contact to form a single daughter droplet, bubble, or particle. Coalescence manifests itself from a microscopic scale in meteorology to a macroscopic scale in astrophysics. For example, it is seen in the formation of raindrops as well as planetary and star formation. In meteorology, its role is crucial in the formation of rain. As droplets are carried by the updrafts and downdrafts in a cloud, they collide and coalesce to form larger droplets. When the droplets become too large to be sustained on the air currents, they begin to fall as rain. Adding to this process, the cloud may be seeded with ice from higher altitudes, either via the cloud tops reaching , or via the cloud being seeded by ice from cirrus clouds. Contrast-enhanced ultrasound in medicine applies microscopic bubbles for imaging and therapy. Coalescence of ultrasound contrast agent microbubbles is studied to pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word '' suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study began in 1845 by Franc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mesoporous Silica
Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and Santa Barbara Amorphous-15, SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and Medical imaging, imaging. Mesoporous ordered silica films have been also obtained with different pore topologies. A compound producing mesoporous silica was patented around 1970. It went almost unnoticed and was reproduced in 1997. Mesoporous silica nanoparticles (MSNs) were independently synthesized in 1990 by researchers in Japan. They were later produced also at Mobil Corporation laboratories and named Mobil Composition of Matter (or Mobil Crystalline Materials, MCM). S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Miscibility
Miscibility () is the property of two substances to mix in all proportions (that is, to fully dissolve in each other at any concentration), forming a homogeneous mixture (a solution). Such substances are said to be miscible (etymologically equivalent to the common term " mixable"). The term is most often applied to liquids but also applies to solids and gases. An example in liquids is the miscibility of water and ethanol as they mix in all proportions. By contrast, substances are said to be immiscible if the mixture does not form a solution for certain proportions. For one example, oil is not soluble in water, so these two solvents are immiscible. As another example, butanone (methyl ethyl ketone) is immiscible in water: it is soluble in water up to about 275 grams per liter, but will separate into two phases beyond that. Organic compounds In organic compounds, the weight percent of hydrocarbon chain often determines the compound's miscibility with water. For examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Amphiphilic
In chemistry, an amphiphile (), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'', nonpolar) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants and detergents. The phospholipid amphiphiles are the major structural component of cell membranes. Amphiphiles are the basis for a number of areas of research in chemistry and biochemistry, notably that of lipid polymorphism. Organic compounds containing hydrophilic groups at both ends of the molecule are called bolaamphiphilic. The micelles they form in the aggregate are prolate. Structure The lipophilic group is typically a large hydrocarbon moiety, such as a long chain of the form CH3(CH2)n, with n > 4. The hydrophilic group falls into one of the following categories: # charged groups #* anionic. Examples, with the lipophilic part of the molecule represented by ''R'', are: #** carboxylates: RCO2− #* ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |