|
Platinum Tetrafluoride
Platinum tetrafluoride is the inorganic compound with the chemical formula . In the solid state, the compound features platinum(IV) in octahedral coordination geometry. Preparation The compound was first reported by Henri Moissan by the fluorination of platinum metal in the presence of hydrogen fluoride. A modern synthesis involves thermal decomposition of platinum hexafluoride. Properties Platinum tetrafluoride vapour at 298.15 K consists of individual molecules. The enthalpy of sublimation is 210 kJmol−1. Original analysis of powdered PtF4 suggested a tetrahedral molecular geometry, but later analysis by several methods identified it as octahedral, with four of the six fluorines on each platinum bridging to adjacent platinum centres. Reactions A solution of platinum tetrafluoride in water is coloured reddish brown, but it rapidly decomposes, releasing heat and forming an orange coloured platinum dioxide hydrate precipitate and fluoroplatinic acid. When heated to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular Prism (geometry), prism with a rectangular Base (geometry), base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum Hexafluoride
Platinum hexafluoride is the chemical compound with the formula Pt F6, and is one of seventeen known binary hexafluorides. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong fluorinating agent and one of the strongest oxidants, capable of oxidising xenon and O2. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers. Synthesis PtF6 was first prepared by reaction of fluorine with platinum metal. This route remains the method of choice. :Pt + 3 F2 → PtF6 PtF6 can also be prepared by disproportionation of the pentafluoride ( PtF5), with the tetrafluoride ( PtF4) as a byproduct. The required PtF5 can be obtained by fluorinating PtCl2: :2 PtCl2 + 5 F2 → 2 PtF5 + 2 Cl2 :2 PtF5 → PtF6 + PtF4 Hexafluoroplatinates Platinum hexafluoride can gain an elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum(IV) Compounds
Platinum is a chemical element; it has symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg, making platinum about 30 times rarer than gold. It occurs in some nickel and copper ores along with some native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable as well as a major precious metal commodity. Platinum is one of the least reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine Trifluoride
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel. Synthesis Bromine trifluoride was first described by Paul Lebeau in 1906, who obtained the material by the reaction of bromine with fluorine at 20 °C: : The disproportionation of bromine monofluoride also gives bromine trifluoride: : Structure Like ClF3 and IF3, the BrF3 molecule is T-shaped and planar. In the VSEPR formalism, the bromine center is assigned two electron lone pairs. The distance from the bromine atom to each axial fluorine atom is 1.81 Å and to the equatorial fluorine atom is 1.72 Å. The angle between an axial fluorine atom and the equatorial fluorine atom is slight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium Tetrafluoride
Selenium tetrafluoride ( Se F4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas. Synthesis The first reported synthesis of selenium tetrafluoride was by Paul Lebeau in 1907, who treated selenium with fluorine Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...: :Se + 2 F2 → SeF4 A synthesis involving more easily handled reagents entails the fluorination of selenium dioxide with sulfur tetrafluoride: :SF4 + SeO2 → SeF4 + SO2 An intermediate in this reaction is seleninyl fluoride (SeOF2). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Tetrafluoride
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid, and later synthesized by John Davy in 1812. It is a tetrahedral molecule and is corrosive. Occurrence Volcanic plumes contain significant amounts of silicon tetrafluoride. Production can reach several tonnes per day. Some amounts are also emitted from spontaneous coal fires.Kruszewski, Ł., Fabiańska, M.J., Ciesielczuk, J., Segit, T., Orłowski, R., Motyliński, R., Moszumańska, I., Kusy, D. 2018 – First multi-tool exploration of a gas-condensate-pyrolysate system from the environment of burning coal mine heaps: An in situ FTIR and laboratory GC and PXRD study based on Upper Silesian materials. Science of the Total Environment, 640-641, 1044-1071; DOI: 10.1016/j.sci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platinum Dioxide
Platinum is a chemical element; it has symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver". Platinum is a member of the platinum group of elements and group 10 of the periodic table of elements. It has six naturally occurring isotopes. It is one of the rarer elements in Earth's crust, with an average abundance of approximately 5 μg/kg, making platinum about 30 times rarer than gold. It occurs in some nickel and copper ores along with some native deposits, with 90% of current production from deposits across Russia's Ural Mountains, Colombia, the Sudbury basin of Canada, and a large reserve in South Africa. Because of its scarcity in Earth's crust, only a few hundred tonnes are produced annually, and given its important uses, it is highly valuable as well as a major precious metal commodity. Platinum is one of the least reactive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group ''Td'', but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram at left, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
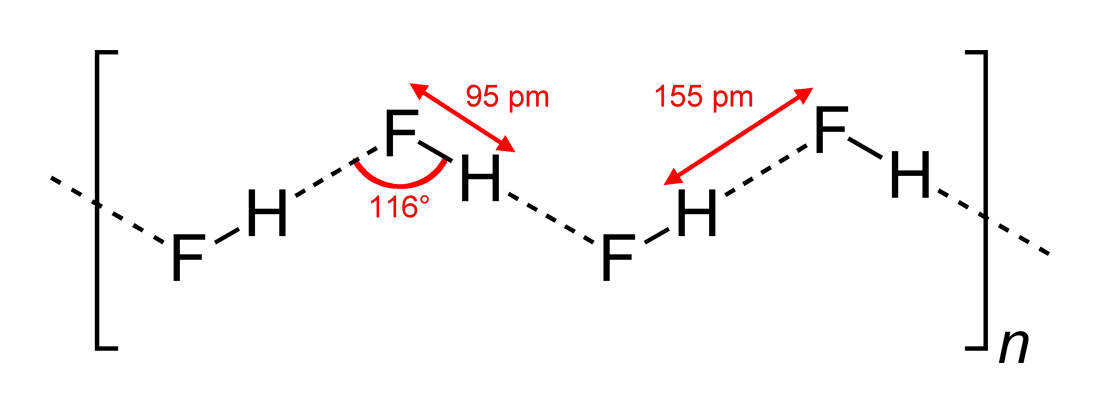
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pearson Symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure. It was originated by William Burton Pearson and is used extensively in Pearson's handbook of crystallographic data for intermetallic phases. The symbol is made up of two letters followed by a number. For example: * Diamond structure, cF8 * Rutile structure, tP6 Construction The two letters in the Pearson symbol specify the Bravais lattice, and more specifically, the lower-case letter specifies the Crystal system, crystal family, while the upper-case letter the Lattice (group), lattice type. The number at the end of the Pearson symbol gives the number of the atoms in the conventional unit cell (atoms which satisfy 1 > x,y,z \geq 0 for the atom's position (x,y,z) in the unit cell). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorination
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for introducing halogens into diverse substrates, e.g. thionyl chloride. Organic chemistry Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more electrophilic and are more aggressive halogenati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |