|
Photoelectrochemistry
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the German electrochemist Heinz Gerischer. The interest in this domain is high in the context of development of renewable energy conversion and storage technology. Historical approach Photoelectrochemistry has been intensively studied in the 1970-80s because of the first peak oil crisis. Because fossil fuels are non-renewable, it is necessary to develop processes to obtain renewable resources and use clean energy. Artificial photosynthesis, photoelectrochemical water splitting and regenerative solar cells are of special interest in this context. The photovoltaic effect was discovered by Alexandre Edmond Becquerel. Heinz Gerischer, H. Tributsch, AJ. Nozik, AJ. Bard, A. Fujishima, K. Honda, PE. Laibinis, K. Rajeshwar, TJ Meyer, PV. Kama ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoelectrochemical Cells
A "photoelectrochemical cell" is one of two distinct classes of device. The first electricity generation, produces electrical energy similarly to a dye-sensitized solar cell, dye-sensitized photovoltaic cell, which meets the standard definition of a solar cell, photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous Electrical conductor, metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water. Both types of device are varieties of solar cells, solar cell, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to Hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
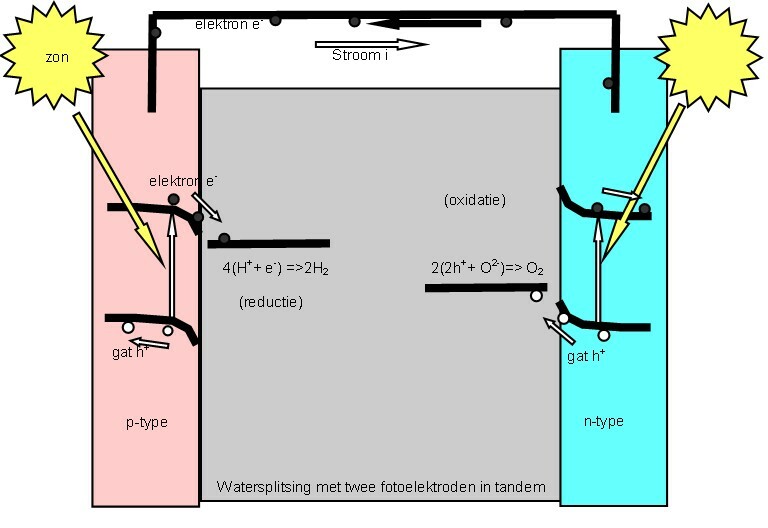
Photoelectrolysis
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the German electrochemist Heinz Gerischer. The interest in this domain is high in the context of development of renewable energy conversion and storage technology. Historical approach Photoelectrochemistry has been intensively studied in the 1970-80s because of the first peak oil crisis. Because fossil fuels are non-renewable, it is necessary to develop processes to obtain renewable resources and use clean energy. Artificial photosynthesis, photoelectrochemical water splitting and regenerative solar cells are of special interest in this context. The photovoltaic effect was discovered by Alexandre Edmond Becquerel. Heinz Gerischer, H. Tributsch, AJ. Nozik, AJ. Bard, A. Fujishima, K. Honda, PE. Laibinis, K. Rajeshwar, TJ Meyer, PV. Kamat, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronically conducting phase (typically an external electrical circuit, but not necessarily, as in Electroless nickel-phosphorus plating, electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic chemical species, species in a Solution (chemistry), solution). When a chemical reaction is driven by an electrical Voltage, potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via the aforementioned electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
John Bockris
Bernhardt Patrick John O’Mara Bockris (5 January 1923 – 7 July 2013) was a South African professor of chemistry, latterly at Texas A&M University. During his long and prolific career he published some 700 papers and two dozen books. His best known work is in electrochemistry but his output also extended to environmental chemistry, photoelectrochemistry and bioelectrochemistry. In the 1990s he experimented with cold fusion and transmutation, topics on which his unorthodox views provoked controversy. Early life John Bockris was born on 5 January 1923, in Johannesburg, South Africa. His mother was Emmeline Mary MacNally and his father Alfred Bockris. He attended a sequence of schools in Brighton, including the preparatory school Withdean Hall from 1934 to 1937, and Xaverian College, a Catholic secondary school, from 1937 to 1940. His father was not present during his childhood: His mother and aunt earned their income from tailoring. University education In 1940 Bockr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Energy
Chemical energy is the energy of chemical substances that is released when the substances undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (2018). "How Batteries Store and Release Energy: Explaining Basic Electrochemistry", ''J. Chem. Educ.'' 95: 1801-1810. http://dx.doi.org/10.1021/acs.jchemed.8b00479 food, and gasoline (as well as oxygen gas, which is of high chemical energy due to its relatively weak double bond and indispensable for chemical-energy release in gasoline combustion). Schmidt-Rohr, K. (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2", ''J. Chem. Educ.'' 92: 2094-2099. http://dx.doi.org/10.1021/acs.jchemed.5b00333 Breaking and re-making chemical bonds involves energy, which may be either absorbed by or evolved from a chemical system. If reactants with relatively weak electron-pair bonds convert to more strongly bonded products ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
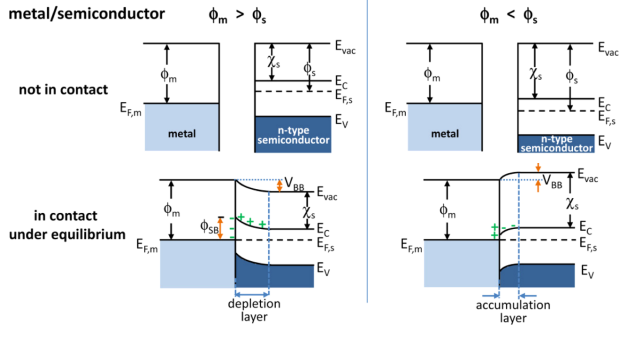
Metal–semiconductor Junction
In solid-state physics, a metal–semiconductor (M–S) junction is a type of electrical junction in which a metal comes in close contact with a semiconductor material. It is the oldest type of practical semiconductor device. M–S junctions can either be rectifying or non-rectifying. The rectifying metal–semiconductor junction forms a Schottky barrier, making a device known as a Schottky diode, while the non-rectifying junction is called an ohmic contact. (In contrast, a rectifying semiconductor–semiconductor junction, the most common semiconductor device today, is known as a p–n junction.) Metal–semiconductor junctions are crucial to the operation of all semiconductor devices. Usually, an ohmic contact is desired so that electrical charge can be conducted easily between the active region of a transistor and the external circuitry. Occasionally, however, a Schottky barrier is useful, as in Schottky diodes, Schottky transistors, and metal–semiconductor fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-type Semiconductor
P-type or type P may refer to: P-type * P-type orbit, type of planetary orbit in a binary system * P-type asteroid, type of asteroid * P-type semiconductor * MG P-type, a type of automobile * P-type ATPase, evolutionarily related ion and lipid pumps * P-Type (rapper), a South Korean rapper Type P * the Audi Type P, a car * a Type P thermocouple See also * For P (and Q) in propositional logic, see modus ponens. {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-type Semiconductor
N-type, N type or Type N may refer to: * N-type semiconductor is a key material in the manufacture of transistors and integrated circuits * An N-type connector is a threaded RF connector used to join coaxial cables * The MG N-type Magnette was produced by the MG Car company from October 1934 to 1936 * The N-type calcium channel is a type of voltage-dependent calcium channel * A Type (model theory) with n free variables * The Dennis N-Type vehicle chassis was used to build fire engines and trucks * The N type carriage The N type carriages are an intercity Passenger car (rail), passenger carriage used on the Rail transport in Victoria, railways of Victoria, Australia. They were introduced between 1981 and 1984 as part of the 'New Deal (railway), New Deal' re ... is an intercity passenger carriage used on the railways of Victoria, Australia * The REP Type N was a military reconnaissance aircraft produced in France in 1914 * N type battery, see: N battery * Type N power plu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Bending
In solid-state physics, band bending refers to the process in which the electronic band structure in a material curves up or down near a junction or interface. It does not involve any physical (spatial) bending. When the electrochemical potential of the free charge carriers around an interface of a semiconductor is dissimilar, charge carriers are transferred between the two materials until an equilibrium state is reached whereby the potential difference vanishes. The band bending concept was first developed in 1938 when Nevill Francis Mott, Mott, Alexander Davydov (physicist), Davydov and Walter H. Schottky, Schottky all published theories of the rectifying effect of Metal–semiconductor junction, metal-semiconductor contacts. The use of semiconductor junctions sparked the computer revolution in the second half of the 20th century. Devices such as the diode, the transistor, the Photoelectric sensor, photocell and many more play crucial roles in technology. Qualitative description ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Level
The Fermi level of a solid-state body is the thermodynamic work required to add one electron to the body. It is a thermodynamic quantity usually denoted by ''μ'' or ''E''F for brevity. The Fermi level does not include the work required to remove the electron from wherever it came from. A precise understanding of the Fermi level—how it relates to electronic band structure in determining electronic properties; how it relates to the voltage and flow of charge in an electronic circuit—is essential to an understanding of solid-state physics. In band structure theory, used in solid state physics to analyze the energy levels in a solid, the Fermi level can be considered to be a hypothetical energy level of an electron, such that at thermodynamic equilibrium this energy level would have a ''50% probability of being occupied at any given time''. The position of the Fermi level in relation to the band energy levels is a crucial factor in determining electrical properties. The Fer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox Potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is expressed in volts (V). Each species has its own intrinsic redox potential; for example, the more positive the reduction potential (reduction potential is more often used due to general formalism in electrochemistry), the greater the species' affinity for electrons and tendency to be reduced. Measurement and interpretation In aqueous solutions, redox potential is a measure of the tendency of the solution to either gain or lose electrons in a reaction. A solution with a higher (more positive) reduction potential than some other molecule will have a tendency to gain electrons from this molecule (i.e. to be reduced by oxidizing this other molecule) and a solution with a lower (more negative) reduction potential w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |